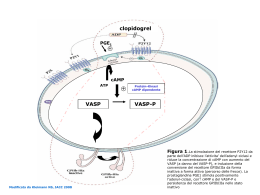
I nuovi farmaci antiaggreganti inibitori del P2Y12 Marco Cattaneo Ospedale San Paolo Università degli Studi di Milano Cumulative Hazard Rates for the First Primary Outcome (Death from Cardiovascular Causes, Nonfatal Myocardial Infarction, or Stroke) during the 12 Months of the Study (patients with ACS, on treatment with aspirin) The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. N Engl J Med 2001;345:494-502 TIENOPIRIDINE METABOLITI ATTIVI N S HOOC HS Cl N Cl Ticlopidine O CH3 N S Cl Clopidogrel O HOOC HS CH3 N Cl agonista ADP ADP specific receptor P2Y12 Gq Gi ++ δ PI3K + stabilizzazione Attivazione/aggregazione piastrinica Cattaneo M, Blood 2011 Ruoli del P2Y12 nell’attivazione/aggregazione piastrinica agonista ADP ADP specific receptor P2Y12 Gq ++ δ prostaciclina IP receptor Gi Gs PI3K AC cAMP + stabilizzazione Attivazione/aggregazione piastrinica Cattaneo M, Blood 2011 AD P agonista O N HOOC ADP SADP S specific receptor P2Y12 Gq Gi δ CH3 Cl prostaciclina IP receptor Gs AC cAMP Attivazione/aggregazione piastrinica Problemi del Clopidogrel • Lento inizio dell’azione farmacologica • Lento recupero della funzione piastrinica dopo sospensione del farmaco (inibizione irreversibile del P2Y12) • Elevata variabilità inter-individuale di risposta Distribution of Platelet Reactivity Units (PRU), measure with VerifyNow P2Y12 in Clopidogrel-treated Patients, by Study and Quartile Brar et al, JACC 2011:58:1945–54 Distribution of Platelet Reactivity Units (PRU), measure with VerifyNow P2Y12 in Clopidogrel-treated Patients, by Study and Quartile ~35% with high on-treatment platelet reactivity (non/poor responders) 230 Brar et al, JACC 2011:58:1945–54 Incidence of MACE for Normal and High On-Treatment Platelet Reactivity: Meta-analysis of Studies of ACS Patients on Treatment with Clopidogrel PRU ≥230 PRU<230 Brar et al, JACC 2011:58:1945–54 Variabili che influenzano la risposta al clopidogrel • Ridotto assorbimento (portatori della mutazione TT3435 del gene ABCB1, che codifica la P-glicoproteina) • Inadeguata attivazione del pro-farmaco in portatori di mutazioni di isoforme del CYP, che ne alterano la funzione enzimatica • Interazione con altri farmaci (PPI, alcune statine, etc) • Età • Indice di massa corporea • Diabete mellito • Fumo di sigaretta • …… • ADERENZA!! Variabilità di risposta al Clopidogrel La soluzione? “Terapia personalizzata”: Aumentare la dose di clopidogrel o usare terapie alternative nei pazienti in cui si dimostri una inadeguata inibizione della funzione piastrinica (in base ai risultati di test di laboratorio) Quale test di funzione piastrinica? • • • • • • • • • Tempo di emorragia Aggregazione piastrinica (aggregometria a trasmissione di luce) Aggregazione piastrinica (ipedenziometria) PFA-100® Verify Now P2Y12 Impact – Cone-and-Plate(let) Analyzer VASP-Phosphorylation TEG …… Quale test «dice la verità»? Paniccia et al, T&H 2010 La «terapia personalizzata» con clopidogrel è efficace e sicura? RECLOSE 2-ACS Incidence of Primary End Point Events at 2y F.U. in ACS patients on DAPT undergoing PCI, according to their platelet reactivity at baseline and F.U. HRPR LRPR P 14.6% 8.7% 0.003 therapy adjustment HRPR at F.U. LRPR at F.U. Parodi et al, JAMA 2011;306:1215-23 RECLOSE 2-ACS Incidence of Primary End Point Events at 2y F.U. in ACS patients on DAPT undergoing PCI, according to their platelet reactivity at baseline and F.U. HRPR LRPR P 14.6% 8.7% 0.003 therapy adjustment HRPR at F.U. LRPR at F.U. 14.9% 14.4% 0.91 Parodi et al, JAMA 2011;306:1215-23 Cumulative Kaplan-Meier Estimates of the Time to the First Adjudicated Occurrence of the Primary Efficacy End Point Price, M. J. et al. JAMA 2011;305:1097-1105 Proportion of patients with primary outcome events Collet et al, NEJM 2012 Cumulative Kaplan-Meier Estimates of the Time to the First Adjudicated Occurrence of the Primary Efficacy End Point Solo 39.8% dei pazienti con UA/ACS!!! Price, M. J. et al. JAMA 2011;305:1097-1105 Proportion of patients with primary outcome events Solo 27% dei pazienti con ACS!!! Collet et al, NEJM 2012 Incidence of MACE for Normal and High On-Treatment Platelet Reactivity: Meta-analysis of Studies of ACS Patients on Treatment with Clopidogrel PRU ≥230 PRU<230 Brar et al, JACC 2011:58:1945–54 Incidence of MACE for Normal and High On-Treatment Platelet Reactivity: Meta-analysis of Studies of ACS Patients on Treatment with Clopidogrel PRU ≥230 PRU<230 Solo 35% dei pazienti con ACS!!! Brar et al, JACC 2011:58:1945–54 RECLOSE 2-ACS Incidence of Primary End Point Events at 2y F.U. in ACS patients on DAPT undergoing PCI, according to their platelet reactivity at baseline and F.U. HRPR LRPR P 14.6% 8.7% 0.003 «Solo» 100% dei pazienti con ACS!!! therapy adjustment HRPR at F.U. LRPR at F.U. 14.9% 14.4% 0.91 Parodi et al, JAMA 2011;306:1215-23 Variabilità di risposta al Clopidogrel Un’altra soluzione? Usiamo farmaci migliori TIENOPIRIDINE METABOLITI ATTIVI N S HOOC HS Cl N Cl Ticlopidine O CH3 N S Cl O HOOC HS CH3 N Cl Clopidogrel O O CH3 O N S O F Prasugrel HOOC HS N F Reletionship between IPA by Clopidogrel 300 mg or Prasugrel 60 mg in response to 20 μM ADP 24 h after the loading dose “Responders” “Non Responders” Brandt et al, Am Heart J 2007 Cumulative Kaplan-Meier Estimates of the Rates of Key Study End Points during the Follow-up Period , in TRITON-TIMI 38 (patients with ACS, scheduled for PCI) Wiviott S et al. N Engl J Med 2007 TICAGRELOR • direct-acting, competitive, reversible P2Y12 • pIC50 of 7.9 with 30 M ADP –induced platelet aggregation (PRP) • Highly selective for P2Y12 (apparently, does not inhibit P2Y1 or other purinergic receptors) • T1/2 7-8.5h (BID drug) • Tmax 2 h DISPERSE Study: Faster and More Consistent IPA With AZD6140 Than With Clopidogrel (Final Extent) Clopidogrel 75 mg qd Day 1 AZD6140 100 mg bid Day 14 Day 1 100 Mean % Inhibition Mean % Inhibition 100 Day 14 80 60 40 20 80 60 40 20 2 4 8 12 2 4 8 Time, h 12 24 2 4 8 12 2 4 8 12 24 Time, h Husted S. Presented at ESC 2005. Cumulative Kaplan-Meier Estimates of the Time to the First Adjudicated Occurrence of the Primary Efficacy End Point in the PLATO trial (patients with ACS, undergoing medical therapy or PCI) HR = 0.84 (0.77–0.92) P<0.001 Wallentin L et al. N Engl J Med 2009;361:1045-1057 Incidence of TIMI-major non-CABG bleeding in studies comparing drugs inhibiting P2Y12 Comparison N. of patients Increase in TIMImajor bleeding P Study Prasugrel vs clopidogrel 13,457 32% 0.03 TRITON-TIMI38, NEJM 2007;357:2001-15 Ticagrelor vs clopidogrel 18,421 25% 0.03 PLATO, NEJM 2009;361:1045-57 Clopidogrel responders vs clopidogrel non-responders 1,608 85% 0.25 Sibbing et al, JACC 2009;53:849-56 Reletionship between IPA by Clopidogrel 300 mg or Prasugrel 60 mg in response to 20 μM ADP 24 h after the loading dose “Responders” “Non Responders” Brandt et al, Am Heart J 2007 DISPERSE Study: Faster and More Consistent IPA With AZD6140 Than With Clopidogrel (Final Extent) Clopidogrel 75 mg qd Day 1 AZD6140 100 mg bid Day 14 Day 1 100 Mean % Inhibition Mean % Inhibition 100 Day 14 80 60 40 20 80 60 40 20 2 4 8 12 2 4 8 Time, h 12 24 2 4 8 12 2 4 8 12 24 Time, h Husted S. Presented at ESC 2005. Incidence of TIMI-major non-CABG bleeding in studies comparing drugs inhibiting P2Y12 Comparison N. of patients Increase in TIMImajor bleeding P Study Prasugrel vs clopidogrel 13,457 32% 0.03 TRITON-TIMI38, NEJM 2007;357:2001-15 Ticagrelor vs clopidogrel 18,421 25% 0.03 PLATO, NEJM 2009;361:1045-57 Clopidogrel responders vs clopidogrel non-responders 1,608 85% 0.25 Sibbing et al, JACC 2009;53:849-56 Differenze tra ticagrelor e prasugrel? (CONFRONTI INDIRETTI!!!) 1. Ticagrelor ha ridotto significativamente la mortalità CV e totale nello studio PLATO (rispetto a clopidogrel) Incidence of death at 12 months in the PLATO trial End point Ticagrelor (n=9,333) Clopidogrel (n=9,291) HR for ticagrelor (95% CI) P value Death from any causes 399 (4.5%) 506 (5.9%) 0.78 (0.69-0.89) <0.001 Death from vascular causes 353 (4.0%) 442 (5.1%) 0.79 (0.69-0.91) 0.001 Death from causes other than vascular causes 46 (0.5%) 64 (0.8%) 0.71 (0.49-1.04) 0.08 Wallentin L et al. N Engl J Med 2009;361:1045-1057 2. Ticagrelor ha aumentato l’incidenza di dispnea in tutti gli studi in cui è stato confrontato con il clopidogrel Comparison of the incidence of dyspnea in patients treated with ticagrelor and patients treated with clopidogrel Study drug Patients (n) Dose Duration A. Percent dyspnea in study group B. Percent dyspnea in clopidogre l group A/B Study Ticagrelor Atherosler (200) 50-400 mg bid 28 d 10-20 0 ∞ DISPERSE Ticagrelor ACS (990) 90 mg bid 180 mg bid 12 wk 12 wk 10.5 15.8 6.4 6.4 1.64 2.47 DISPERSE 2 Ticagrelor Stab. CAD (123) 90 mg bid 6 wk 38.6 9.3 4.15 ONSET/ OFFSET Ticagrelor Stab. CAD (98) 90 mg bid 14 d 13 4 3.25 RESPOND Ticagrelor ACS (18,624) 90 mg bid 12 mo 13.8 7.8 1.77 PLATO Ticagrelor e dispnea • Nella maggior parte dei casi, la dispnea era insorta entro I primi 7 giorni dall’inizio della terapia, era lieve/moderata, si risolveva spontaneamente • Studi approfonditi hanno dimostrato che la dispnea associata con l’uso del ticagrelor NON era causata da – disfunzione polmonare – disfunzione cardiaca – acidosi Storey et al, JACC 2010; Am J Cardiol 2011; Eur Heart J 2011 TICAGRELOR Effetti «Off-target» Residual adenosine concentrations, measured in whole blood by LC-UV over time, after the addition of 7.1μmol/L adenosine Dipyridamole ( ) Ticagrelor ( ) PAM ( ) DMSO (X) Meccanismi di inibizione della funzione piastrinica da parte del ticagrelor Nylander et al, JTH 2013 Effetto del ticagrelor sull’adenosina • Spiega l’effetto del farmaco sulla mortalità? Effetto del ticagrelor sull’adenosina • Spiega l’effetto del farmaco sulla mortalità? • Spiega l’aumento della dispnea associato con l’uso del farmaco? Rappresentazione schematica delle vie afferenti della dispnea dai recettori vagali e da chemorecettori periferici al SNC Burki, Chest 2010 Dispnea e farmaci antiaggreganti P2Y12 inhibition Adenosine increase Dyspnea Ticagrelor YES (reversible) YES YES Cangrelor YES (reversible) NO YES Elinogrel YES (reversible) NO YES Dipyridamole NO YES NO Cilostazol* NO YES NO Clopidogrel YES (irreversible) NO YES** * D. Angiolillo: personal communication ** albeit to a much lower extent than ticagrelor, elinogrel and cangrelor Dispnea e farmaci antiaggreganti P2Y12 inhibition Adenosine increase Dyspnea Ticagrelor YES (reversible) YES YES Cangrelor YES (reversible) NO YES Elinogrel YES (reversible) NO YES Dipyridamole NO YES NO Cilostazol* NO YES NO Clopidogrel YES (irreversible) NO YES** * D. Angiolillo: personal communication ** albeit to a much lower extent than ticagrelor, elinogrel and cangrelor Inibitori del recettore P2Y12: reversibili vs irreversibili Cellule che esprimono il P2Y12 • • • • • • • Piastrine Cellule gliali Neuroni Cellule muscolari lisce Leucociti Macrofagi … Thienopyridines Time (h) Plasma drug concentration Inhibition of P2Y12 on platelets Inhibition of P2Y12 on nucleated cells Cattaneo, Thromb Haemost 2012 Thienopyridines Reversible P2Y12 inhibitors Time (h) Time (h) Plasma drug concentration Inhibition of P2Y12 on platelets Inhibition of P2Y12 on nucleated cells Cattaneo, Thromb Haemost 2012 Conclusioni - 1 • L’inibizione della funzione piastrinica da parte dei «nuovi» farmaci anti-P2Y12, prasugrel and ticagrelor, è meno variabile ( mediamente maggiore), rispetto al clopidogrel • I «nuovi» P2Y12 antagonisti hanno un rapporto rischio/beneficio più favorevole del clopidogrel • Pertanto, i «nuovi» P2Y12 antagonisti rappresentano una valida soluzione al problema della variabilità di risposta al clopidogrel Conclusioni - 2 • Ticagrelor inibisce la funzione piastrinica anche aumentando la concentrazione plasmatica di adenosina • Alcuni effetti clinici (sia benefici che avversi) del ticagrelor potrebbero essere mediati da: – aumento della concentrazione plasmatica di adenosina – inibizione permanente delle funzioni P2Y12dipendenti in cellule nucleate Conflitti di interesse • Supporto per la ricerca e onorari: AstraZeneca, Eli Lilly, Daiichi-Sankyo, Evolva • Onorari: Sanofi • Dirigo un laboratorio per lo studio della funzione piastrinica nell’Ospedale in cui opero
Scaricare