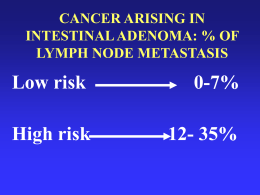
XV Congresso Nazionale SIGU Carcinoma Gastrico Ereditario: Strategie Terapeutiche Maria Di Bartolomeo Divisione di Oncologia Medica INDICE • Epidemiologia e sopravvivenza • Terapia adiuvante – – Chemioradioterapia Chemioterapia adiuvante – Studio – Analisi biologica ITACA-S • Approccio neoadiuvante e perioperatorio • Nuova classificazione molecolare • Terapia biologica Gastric cancer: a global disease • • • • • 4th most common malignant disease ~ 930,000 2nd most common cause of cancer-related death worldwide ~700,000 Falling incidence of distal gastric cancer Increasing incidence of proximal gastric cancer Wide geographical variation Incidence (males) 20 / 100 000 10 - 20 / 100 000 <10 / 100 000 www.cancer.gov Kamangar F et al. J Clin Oncol 2006;24:2137–50 EUROCARE-4: Sopravvivenza dei Pazienti con Carcinoma Gastrico. Dal 1995-1999 European Journal of Cancer 45 (2009) Incidenza in Italia: tasso grezzo rapporto tra casi e popolazione Sardegna Sicilia Puglia Uomini Donne Campania Calabria Abruzzo Lazio Lombardia Emilia Romagna Toscana M arche Umbria 0 5 10 15 20 25 30 35 40 45 50 Stimati dal Reparto Epidemiologia dei Tumori di Epidemiologia Sorveglianza e Promozione della Salute dell’ISS Which advice for patients with familial predisposition? HP screening and eradication In Japan & Korea: gastroscopy every year in patients with familial incidence Genetic counseling Search for Ecadherin in families with ≥ 2 (with diffuse type gastric cancer), esp. at younger age Gastroscopy every 1-2 years (some specified after age 40) in Ecadherin mutation carriers Consider gastrectomy in selected patients with Ecadherin mutation Type of surgical procedure in resectable gastric cancer Extent of resection partial vs total: depending on location, margins (> 5 cm) and diffuse type/signet cells partial gastrectomy for distal cancer (antrum) Diffuse type/ signet cells: total gastrectomy histology: Extent of resection: D2 preferred: better long term outcome Splenectomy: not done, unless direct invasion laparoscopic resection: clinical trials Reconstructive techniques (pouch): better QOL Improved outcome in high volume centers - centralisation Neo-adjuvant and adjuvant therapy for gastric cancer: different strategies Post-operative Chemoradiotherapy (trend to perioperative CT in academic centers) Peri-operative Chemotherapy (ECF- 5FU/cisplatin) Postoperative CT Post-operative Chemotherapy (S-1 or combination) Chemioradioterapia adiuvante 8/1991-6/1998: 556 pz (275 trattamento perioperatorio,281 chirurgia) Chirurgia radicale R FU/LV (1 ciclo) FOLLOW UP Radioterapia FU/LV(2 cicli) FU/LV (2 cicli) (Macdonald, NEJM, 2001) Bajetta, Cascinu, Di Costanzo, De Vita, Ann Oncol, 2002 JNCI, 2007 JNCI, 2008 Ann Oncol, 2007 Stage T3-4/N+ T3-4/N+ T3-4/N+ I-IIIB Pts 137/137 196/201 128/130 112/113 Experimental Arm EAPFU/LV PELFwk PELF ELFE Control arm Follow-up FU/LV Follow-up Follow-up HR 0.93 0.95 0.90 0.91 5-y OS control arm 49% 50% 48.7% 43.5% ITACA-S “Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropirimidine “ 10/2001-12/2004: 1059 pz (529 CT, 530 follow-up) Resezione gastrica R S-1 x 1 anno Follow-up S-1 : 80mg/m2 /die (Sakuramoto, NEJM 2007) Mono CT vs Controllo (N=324 dai 2 studi): Curva di Kaplan-Meier HR=0.60 (p=0.03; 95% CI:0.42,0.84) 5 anni OS : 54% versus 69% nel gruppo mono-CT 15 Comparison of a sequential treatment with irinotecan (CPT-11) plus 5-fluorouracil (5FU)/folinic acid (LV) followed by docetaxel and cisplatin versus a 5-FU/LV regimen as postoperative treatment for radically resected gastric cancer E.Bajetta, I.Floriani, M.Di Bartolomeo, R.Labianca, A. Santoro, R.Casaretti, E.Pasquini, F.Di Fabio, G.Pinotti, P.Bidoli, G.Rosati, A.Mambrini, A.Ciarlo, S.Ricci, L. Frassinetti, F.Di Costanzo, AM.Bochicchio on behalf of ITACA-S group PRESENTED BY MARIA DI BARTOLOMEO Not for profit, multicenter, randomized, parallel arm, superiority trial Adenocarcinoma of the stomach or GEJ Stratified for center and lymph-nodal involvement (N-/N+) Experimental Experimental armarm CPT-11 :180 mg/m2 die 1 5-FU: 400-600 mg/m2 die 1,2 LV :100 mg/m2 die 1,2 Q2wks, 4 administrations Control arm 5-FU: 400-600 mg/m2 die1,2 LV: 100 mg/m2 die 1,2 Q2wks, 9 administrations TXT:75 mg/m2 die 1 CDDP: 75 mg/m2 die 1 Q3 wks, 3 administrations ITACA-S 1106 Randomized 541 control arm 565 experimental arm 6 pts excluded due to major violations: 3 control arm 3 experimental arm 6 pts crossed group 1100 ITT population 538 control arm 562 experimental arm 28 pts never started treatment: 4 pts from control to experimental 2 pts from experimental to control 16 control arm 12 experimental arm 1072 Safety population 520 control arm 552 experimental arm HR:0.98 95%CI: 0.83-1.16 p=0.83 Median DFS: 41.3 months 5-year DFS: 44.8% ITACA-S HR:1.0 95%CI: 0.83-1.20 p=0.986 Median OS: 69.8 months 5-year OS: 52.2% ITACA-S BIOLOGICAL STUDY: FLOW CHART Pathological disease stage, node involvement, histological type, tumor site (distal or proximal), history of H.pylori infection Selected Patients from 25 centers Primary tumor tissue sample from gastric surgery Formalin-fixed paraffin-embedded (FFPE) tissue. Pathological diagnosis Immunohistochemical staining COX-2, E-cadherin, ßcatenin and osteopontin proteins Fluorescence in situ ibridization HER-2 amplification Mutational analysis DNA extraction PI3KCA, BRAF, KRAS mutations, MSI Giemsa stains on normal mucosa H. pylori Disease-free interval and overall survival Molecular mechanisms of ß-catenin and E-cadherin in gastric cancers • E-cadherin regulates adesion, migration and differentiation • ß-catenin regulates adhesion, migration and nuclear transcription Molecular mechanisms of osteopontin (OPN) and COX-2 in gastrointestinal cancers • OPN signaling results in various functions, including prevention of apoptosis, modulation of angiogenesis, degradation of extracellular matrix, activation of PI3KCA and NG-κB pathways • COX-2 is involved in angiogenesis and inflammation ASSOCIATION OF BIOMARKERS AND PATHOLOGICAL VARIABLES • E-Cadherin was significantly associated with diffuse type and poorly differentiated tumors (p=0.02) • OPN was significantly associated with pTNM (p=0.02) Biomarkers distribution % of samples Osteopontin score • negative • positive 54% 46% Cox score • low • high 92% 8% Beta-catenin • Normal membranes • Cytoplasmic / loss membranes • Any nuclear 62% 28% 10% E-cadherin • Normal membranes • Cytoplasmic / loss membranes • Any nuclear 65% 28% 7% Osteopontin RELAPSE FREE SURVIVAL, RFS 5-year RFS (95% confidence interval) OPN negative 49.2% (41.2,58.8%) OPN + / ++ 32.6% (25.1,42.2%) Log-rank test p<0.001 Time, months Nuclear ß-catenin/E-cadherin 5-year RFS (95% confidence interval) 43.2% (37.0,50.3%) Nuclear expression 33.2% (21.4,51.7%) RELAPSE FREE SURVIVAL, RFS All others Log-rank test p=0.04 Time, months Trattamento pre-operatorio perchè’? • potenziale beneficio di un trattamento pre-operatoro • incremento chirurgia R0 • trattamento delle micrometastasi • valutazione chemiosensibilità del tumore “Perioperative Chemotherapy in Operable Gastric Cancer. Final Results of Randomized Trial” 7/1994-4/2002: 530 pz (250 CSC, 253 S) • chirurgia non standardizzata R ECF X 3 Chirurgia • Probabilità di sopravvivenza 5 anni: CSC : 36% (30%-43%) S: 23% (17%- 29%) Chirurgia HR: 0.75 (95% CI:0.60-0.93 P=0.009) ECF X3 Follow up (Cunningham, NEJM, 2006) Role of HER2 in Gastric Cancer clinicaloptions.com/oncology Molecular Targets: Esophagogastric Cancer KRAS mutation: < 5% to 10%[1,2] BRAF mutation: < 5%[1,2] EGFR overexpression: ~ 50% to 80%[3,4] – TKIs inactive[4] – Cetuximab monotherapy inactive[5] EGFR mutation: very low[4,6] HER2 overexpression: 10% to 25%[7] 1. Lee SH, et al. Oncogene. 2003;22:6942-6945. 2. Kim IJ, et al. Hum Genet. 2003;114:118-120. 3. Galizia G, et al. World J Surg. 2007;31:1458-1468. 4. Dragovich T, et al. J Clin Oncol. 2006;24:4922-4927. 5. Chan JA, et al. Ann Oncol. 2011;[Epub ahead of print]. 6. Mammano E, et al. Anticancer Res. 2006;26:3547-3550. 7. Yano T, et al. Oncol Rep. 2006;15:65-71. Role of HER2 in Gastric Cancer clinicaloptions.com/oncology HER2 Positivity Histological Type Histological type Localization Subtype HER2 Positivity (%) P Value Intestinal Diffuse Mixed 32.2 6.1 20.4 < .001 GEJ Gastric 33.2 20.9 < .002 Histological subtype and tumor sublocalization are important factors for HER2 expression/amplification in gastric cancer Bang YJ, et al. ASCO 2009. Abstract 4556. Reprinted with permission Role of HER2 in Gastric Cancer clinicaloptions.com/oncology Survival Probability ToGA: OS in IHC 2+/FISH+ or IHC 3+ (Exploratory Analysis) 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 Median Events OS HR FC + T FC 11.8 0 2 4 6 120 136 16.0 11.8 95% CI 0.65 0.51-0.83 16.0 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 Mos Pts at Risk, n 228 218 196 170 142 122 100 84 218 198 170 141 112 96 75 53 65 39 51 28 39 20 28 13 20 11 12 4 11 3 5 3 4 0 1 0 0 0 Reprinted from The Lancet, 376(9742), Bang YJ, et al., Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. 687-697, Copyright 2010, with permission from Elsevier. Role of HER2 in Gastric Cancer clinicaloptions.com/oncology Median OS Increased to > 1 Yr With Trastuzumab-Based Therapy BSC[1] 12 mos FAMTX[2] C + S1[3] CF[4] IF[5] EOF[6] DCF[4] ECF[6] ECX[6] XP[7] EOX[6] Trastuzumab + XP/FP[8] HER2 IHC 2+/FISH+ or IHC 3+ 15 10 5 0 Median OS in Patients With Advanced Gastric Cancer (Mos) 1. Murad AM, et al. Cancer. 1993;72:37-41. 2. Vanhoefer U, et al. J Clin Oncol. 2000;18:2648-2657. 3. Ajani JA, et al. J Clin Oncol. 2010;28:1547-1553. 4. Van Cutsem E, et al. J Clin Oncol. 2006;24:4991-4997. 5. Dank M, et al. Ann Oncol. 2008;19:1450-1457. 6. Cunningham D, et al. N Engl J Med. 2008;358:36-46. 7. Kang YK, et al. Ann Oncol. 2009;20:666-673. 8. Bang YJ, et al. Lancet. 2010;376:687-697. Acknowledgments A.O. C. Poma – Mantova (Dr Aitini A.O. Parma – Parma (Dr ssa Pucci- Dr Camisa) A.O. Padova- (Prof Nitti- Prof Rugge) A.O. Santa Maria Nuova - Reggio Emilia (Dr Boni) A.O. San Carlo - Potenza (Dr Rosati- Dr Bilancia) A.O. S.Orsola-Malpighi - Bologna (Dr Martoni) A.O. Universitaria Careggi- Firenze (Prof. Di Costanzo) Azienda USL 6 - P.O. di Livorno - Livorno (Dr Cappuzzo) Fondazione IRCCS Istituto Nazionale- Milano (Dr.ssa Di Bartolomeo- Dr Pellegrinelli) Fondazione Poliambulanza- Brescia (Dr Zaniboni) G.B. Morgagni-L. Pierantoni- Forlì (Prof Amadori) I.E.O. - Milano (Dr Fazio- Dr.ssa Lombardi) Istituto Clinico Humanitas - Rozzano (MI) (Dr Santoro- Dr ssa Rubino) Ospedale Civile “Guglielmo Saliceto” –Piacenza (Dr Cavanna) Ospedali Riuniti- Bergamo (Dr Labianca) Ospedale Civico - Carrara (Dr Cantore) Ospedale degli Infermi - Rimini (Dr Ravaioli) P.O. Serbelloni - Gorgonzola (MI) (Dr Venezia) Università di Sassari – Sassari (Prof Farris) Dr Filippo Pietrantonio Prof Giuseppe Pelosi Dr Alessandro Pellegrinelli Dr. Giovanni De Luca Dr.ssa Irene Floriani Dr. Davide Poli Sig. Monica Cropolato Dr.ssa Eliana Rulli Anatomia Patologica INT Istituto Mario Negri
Scaricare