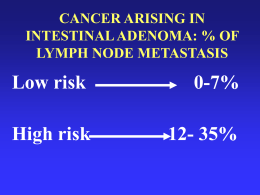
Struttura Complessa di Ginecologia Oncologica Direttore: Prof. Stefano Greggi Carcinoma della Cervice Uterina Cronoprogramma Diagnostico-Terapeutico Pap-test Anormale L-SIL H-SIL Bethesda System, 2001 Pap-test Anormale Pap-test Anormale H-SIL 8% ICC 0% L-SIL 31% ASC-US 61% Davey, 2004 ASC-US INCIDENCE: 1.3-5.0% CYTOLOGIC REVISION • Low reproducibility level • Low PPV NEGATIVE RISK OF CIN2+ RISK OF CIN3+ 75-85% 12% 5% Downgraded to neg 40% Upgraded to L-SIL 20% Upgraded to H-SIL 2% Solomon (ALTS Group), 2001 Stoler, 2001 Sherman, 2001 Kristen (ALTS Group), 2006 % Upgrading CIN 2-3 Cancer Microinv. ASC-US ASC-H CIN 3 Inv. 5-17 0.2 24-94 6-12 1-2 ASC-US – HPV-test Triage HPV-test HR + HR - Colposcopia Pap-test a 12 mesi + Colposcopia - Screening SICPCV, 2006 HPV-test Triage – Raccomandazioni Statement on HPV DNA test utilization, 2009 p16 Triage (sperimentale) HPV-test (screening) HR + HR - p16-test + Colposcopia - HPV-test a un anno Carozzi, 2008 ASC-US - ASC-H - L-SIL SICPCV, 2006 H-SIL – Carcinoma squamocellulare SICPCV, 2006 AGC SICPCV, 2006 Follow-up • Citologia e colposcopia ogni 6 mesi per 2 anni • Controllo annuale per altri 5 anni • Ritorno a screening A 6 mesi da trattamento Colposcopia, citologia e HPV-test Colposcopia e/o citologia HPV + Controllo a 6 mesi - Colposcopia e/o citologia + Pap-test e HPV-test a 12 mesi Percorso sec. lesione + Colposcopia - Screening SICPCV, 2006 Istotipi • Carcinoma squamoso in situ • Carcinoma squamoso inf. ~80% cheratinizzante, non-cheratinizzante, verrucoso • Adenocarcinoma in situ / tipo endocerv. • Adenocarcinoma endometrioide • Adenocarcinoma a cellule chiare • Ca. adenosquamoso • Ca. adenoide cistico • Ca. a piccole cellule • Ca. indifferenziato • Ca. neuroendocrino ~10% FIGO, 2006 Cervical Cancer - FIGO Staging (2009) I IA IA1 IA2 IB IB1 IB2 II IIA IIA1 IIA2 IIB III IIIA IIIB IV IVA IVB The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded) Invasive carcinoma which can be diagnosed only by microscopy, with deepest invasion ≤5mm and largest extension ≤7mm Measured stromal invasion ≤3mm in depth and horizontal extension ≤7mm Measured stromal invasion >3mm and not >5mm with an extension of not >7mm Clinically visible lesions limited to the cervix or pre-clinical cancers > Stage IA Clinically visible lesion ≤4cm in greatest dimension Clinically visible lesion >4cm in greatest dimension Cervical carcinoma invades beyond the uterus, but not to the pelvic wall or to the lower third of the vagina Without parametrial invasion Clinically visible lesion ≤4cm in greatest dimension Clinically visible lesion >4cm in greatest dimension With obvious parametrial invasion The tumor extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or non-functioning kidney Tumor involves lower third of the vagina (No extension to the pelvic wall) Extension to the pelvic wall and/or hydronephrosis or non-functioning kidney The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to Stage IV Spread of the growth to adjacent organs Spread to distant organs Microinvasive CC • IA Early CC • IB1 • IIA1 Locally Advanced CC (LACC) • IB2 Metastatic CC • IIA2 • IIB • III • IVA • IVB CONIZZAZIONE CERVICALE EVISCERAZIONE PELVICA Microcarcinoma – Staging Criteria FIGO IA1: stromal invasion ≤ 3 mm in depth, horizontal extension ≤ 7 mm IA2: stromal invasion 3-5 mm in depth, horizontal extension ≤ 7 mm SGO Stromal invasion ≤ 3 mm in depth, no LVSI Microcarcinoma – Treatment • Total abdominal or vaginal hysterectomy (if VAIN, appropriate cuff of vagina should be removed) IA1 • Observation after cone biopsy (particularly if fertility is desired) • Modified RH (Type 2) and pelvic LND • Consider extrafascial H and pelvic LND (if no LVSI) IA2 If fertility is desired: • large cone biopsy + extra-perit. or lpsc pelvic LND • rad. trachelectomy and extra-perit.or lpsc pelvic LND Follow-up Mainly with Pap smears annually after two normal smears at 4 and 10 mos FIGO, 2006 Cone: Positive margin Microcarcinoma – Cone Positive Margin In patient with positive margins: • Vaginal Strict Follow-Up • Endocervical or Stromal Repeat Conization or Hysterectomy Fertility-sparing surgery Cervical Cancer 43% of cervical cancer in women <45y (10-15% during childbearing years) Radical Trachelectomy • Vaginal • Abdominal • Laparoscopic • Robotic Eligibility criteria • Age < 40-45 years & Strong fertility desire • Diagnosis of invasive cancer (ideally, disease located primarily on the ectocervix) • Exclusion of unfavorable histology • Stage IA1 with LVSI, IA2, IB1<2 cm • No evidence of pelvic N met and/or distant met • Gynecologic oncologist experienced in laparoscopic and radical vaginal surgery Dargent, 1994 Fertility-sparing surgery RVT & Cancer prognosis Review n Recurrence Rates Death Rates Darsun, 2007 520 4.2 2.8 Sonoda, 2008 548 4.0 2.6 Plante, 2008 603 4.5 2.5 Overall recurrence and death rates comparable to early-stage cervical cancer treated by RH or RT Plant, 2004; Seli, 2005 Fertility-sparing surgery RVT & Pregnancy outcome Review Review (8 studies : 603 RVT / 256 pregnancies) (16 studies: 355 RVT / 161 pregnancies) Pregnancy rate 62% Pregnancy rate 70% TAB/EUP 1st-2nd trimester loss Deliveries <32 ws 5% 27% 12% 1st-2nd trimester loss 30% Deliveries >37 ws Currently pregnant 65% 6% Plante, 2008 Boss, 2005 Cerv Microca – Conservative Treatment Algorythm CK Conization IA2 Margins - Follow-up LVSI + Margins + No Res T Repeat cone LVSI Invasive Res T Pelvic LND RH N+ N- Follow-up RH + pelvic LND CERVICAL CARCINOMA Clinical Assessment T size Lymphnode mets Vaginal infiltration FIGO Stage Histotype & Grade Bladder/Rectum involvement Parametrial infiltration Stadiazione Clinica • Esame vaginale bimanuale e vagino-rettale (in narcosi) • Colposcopia, biopsia / conizzazione • Currettage endocervicale • Cistoscopia • Retto-sigmoidoscopia • Rx torace (2 proiezioni) • TAC/RMN (PET) CC apparentemente iniziale • RX torace • RMN addome/pelvi CC localmente avanzato • Visita ginecologica in narcosi • RX torace • RMN addome/pelvi • Uretrocistoscopia • Retto-sigmoidoscopia FIGO, 2006 Cervical Cancer Comparison of Diagnostic Procedure Utilization ACRIN 6651/GOG 183 (n=208 ;Stage ≥ IB) 1978 1983 1988-1989 2002 Cystoscopy 64% 80% 52% 8.1% Sigmoidoscopy 44% 58% 49% 8.6% Barium enema 58% 60% 32% 0 Intravenous urogram 86% 91% 42% 1.0% Lymphangiography 18% 11% 14% 0 CT/MRI 16% 54% 70% 100% Montana, 1995 Amendola, 2005 Cervical Cancer MRI MRI staging for cervical cancer is now widely accepted as an optimal method for evaluation of tumor volume, uterine corpus involvement, parametrial invasion, … Narayan K, 2003 … but prediction of parametrial, bladder and rectal involvement is correct in 75% of cases at best Bipatt, 2003 Narayan, 2005 Follen, 2003 Cervical Cancer Detection of Advanced Stage (>IIB) Cancer by Retrospective Readers of CT & MRI ACRIN 6651/GOG 183 (n=208 – Stage ≥ IB) CT MRI P Value Mean sensitivity (%) 28 47 0.104 Mean specificity (%) 90 79 0.099 Mean PPV (%) 55 36 0.001 Mean NPV (%) 83 85 0.305 Hricak, 2007 Cervical Cancer Performance of CT & MRI in Detecting Lymph Node Involvement ACRIN 6651/GOG 183 (n=208 – Stage ≥ IB) CT MRI Sensitivity (%) 31 37 Specificity (%) 86 94 Hricak, 2005 Treatment – Stage IB1, IIA1 • Modified RH (Type 2) or RH (Type 3) and pelvic LND • Adjuvant pelvic RT plus BRT • Adjuvant concurrent CTRT (Cisplatin±5FU) ↑ survival in such patients In younger patients, if post-operative radiation is likely to be given: • ovaries may be preserved and suspended outside the pelvis FIGO, 2006 Treatment – Stage IB1-IIA1 • RH tipo III + LA pelvica + sampling N aortici • RT pelvi + BRT Se desiderio di prole (solo per IB1): • trachelectomia radicale + LA pelvica ± sampling N aortici NCCN, 2009 Radical Hysterectomy – History & Classification Wertheim (1900) Okabayashi (1921) Meigs (1951) Piver-Rutledge (1974) Nerve-sparing (1990s) Mota-EORTC (2008) Robotics (2000s) Querleu-Morrow (2008) Radical Hysterectomy – Piver-Rutledge Classification • Type I (Extrafascial hysterectomy): simple hysterectomy to remove the entire cervical tissue • Type II (Modified RH): basically, the RH described by Wertheim, to remove more paracervical tissue while still preserving the blood supply to the distal ureters and bladder • Type III (RH): first described by Meigs in 1944, with the purpose of a wide excision of parametrial and paravaginal tissue • Type IV (Extended RH): complete removal of the periureteral tissue and a more extensive resection of the paravaginal tissue • Type V (Partial exenteration): radical removal of disease involving the distal ureter and/or bladder Piver, 1974 THE POINT OF TRANSECTION OF THE UTEROSACRAL AND CARDINAL LIGAMENTS IN CLASS II AND III RH Type 3 RH Type 3 RH Type 2 RH Radical Hysterectomy – Querleu-Morrow Classification • Type A (Minimum resection of paracervix): extrafascial hysterectomy, corresponds to the type I RH, with a <10 mm vaginal resection • Type B (Transection of paracervix at the ureter): corresponds to the type II RH, with (B2) or without (B1) additional removal of the lateral paracervical lymph nodes, and >10mm vaginal resection • Type C (Transection of paracervix at junction with internal iliac vascular system): corresponds to type III RH, with the ureter completely mobilized, 15-20mm of vagina and corresponding paracolpos resected routinely; with (C1) or without (C2) autonomic nerve preservation • Type D (Laterally extended resection): ultraradical procedures mostly indicated at the time of pelvic exenteration, with the entire paracervical resection at the pelvic sidewall including the hypogastric vessels (D1); type D2 includes the resection of adjacent fascial-muscular structures Querleu, 2008 Quality control and results comparison in RH The term paracervix replaces others such as cardinal or Mackenrodt’s ligament, or parametrium, and includes that usually named as paracolpium It is recommended to include the following information in the operative report: • All parts defining the type of RH (transection of paracervix and vagina, uterine artery) • Surgical (fresh sample) and pathological (fixed sample) length of ventral, dorsal and lateral extent of paracervix resection • Surgical/pathological minimum length of vagina resected • Minimum distance between tumor and resection margins (when applicable) Querleu, 2008 Type A Type B1 Type C2 Surgery-related Complications Rad. Hysterectomy (type III) + Pelvic Lymph. 10-15% Severe Perioperative Compl. 20-30% Early/Late Bladder/Rectal Disf. 75% vs 10% (III vs II) Temp. Bladder Disf. Literature Review LN Involvement by Stage FIGO, 2006 Treatment – Stage IB2, IIA2 • Primary CTRT • Primary RH and pelvic LND + Adjuvant RT • Neoadjuvant CTRT (3 courses of platinum based CT) + RH and pelvic LND ± Adjuvant post-operative CT or RT If positive common iliac or paraaortic nodes: • extended field radiation should be considered FIGO, 2006 Treatment – Stage ≥ IIB • Primary CTRT (RT plus BRT) • Primary pelvic exenteration (Stage IVA not involving pelvic sidewall) If positive common iliac or paraaortic nodes: • extended field radiation should be considered IIB-IVA • Primary CT (Cisplatin) Unclear impact of CT on palliation and survival IVB FIGO, 2006 Treatment – Stage IB2-IVA • RH tipo III + LA pelvica + sampling N aortici IB2-IIA2 • CTRT (RT pelvi + Cisplatino + BRT) • CTRT (RT pelvi + Cisplatino + BRT) + isterectomia adiuvante IIB-IVA • CTRT (RT pelvi + Cisplatino + BRT) NCCN, 2009 Terapia Adiuvante & Follow-up N pelvici + Margini + Parametrio + N- RT pelvi + CT(P) ± BRT (margini vaginali +) • RT pelvi (volume, invasione stromale, LVSI) ± CT(P) • Follow-up EO gen & gin Pap-test • ogni 3 mesi (1° anno) • ogni 4 mesi (2° anno) • ogni 6 mesi (3-5° anno) • annuali (> 6° anno) Rx Torace ogni anno (opzionale) Laboratorio ogni 6 mesi (opzionali) CT/MRI/PET su indicazione clinica NCCN, 2009 (Neo)adjuvant Setting NACT – Rationale NACT SHRINKAGE OF PRIMARY TUMOR TREATMENT OF LOCO-REGIONAL AND DISTANT MICROMETASTASES ADDITIONAL LOCAL TREATMENT BETTER DISEASE CONTROL SURVIVAL BENEFIT NACT + Surgery vs Exclusive RT (LACC) Italian Multicenter Randomized Study, 2001 Stage IB2-IIB Stage III NACT & Radical Surgery (Locally Advanced Cervical Cancer) Review & Meta-analysis Endpoint Survival DFS Loco-regional DFS Metastases-free survival Nr. of events / patient 368/872 414/872 402/872 381/872 HR (p value) 0.65 (0.00004) 0.68 (0.0001) 0.68 (0.0001) 0.63 (0.00001) The absolute improvement in survival of 15% (8-21%) at 5years obtained by NACT is of the same magnitude as that achieved with the standard cisplatin-based CTRT Cochrane Coll., 2009 EORTC Trial 55994 Study Coordinators: S. Greggi G. Kenter F. Landoni Cervical Cancer (age 18-75) IB2; IIA2; IIB RANDOM NACT + Radical Surgery Exclusive CTRT Flow-Chart Sospetto K cervice uterina Biopsia cervicale Ca invasivo Ca microinvasivo Conizzazione Cervicale Stadiazione clinica RMN addome / pelvi Colposcopia, Rx torace, SCC Ag, Visita gin. in narcosi, Cistoscopia e Rettoscopia IB1 IR tipo B o C + LA pelvica o CTRT MRC Parametri N- FU IB2 - II III - IVA CTNA + IR tipo C + LA pelvica o CTRT CTRT o Pelvectomia + LA pelvica MRC + parametri + N+ Inf stroma cerv >90% RT CT +/- RT Ca non definito / CIN III Ca invasivo Ca microinvasivo IVB IA1 (margini -) CT sistemica FU IA2 Vedi algoritmo dedicato Follow-up Carcinoma della Cervice non Radiotrattato 1° e 2° anno 3° e 4° anno Ogni 6 mesi Ogni 6 mesi Ogni 12 mesi 5° anno > 5° anno Ogni 12 mesi Ogni 12 mesi A 30 gg Ogni 3 mesi Visita ginecologica X X X X X E.O. generale X X X X X Colposcopia X X X X Pap-Test X X X X Rx torace X X X RMN addome-pelvi* X X X Urinocoltura (+ ev. Abg) X X X CA125 X X X SCC X X X Follow-up Carcinoma della Cervice Radiotrattato 1° e 2° anno 3° e 4° anno Ogni 6 mesi Ogni 6 mesi Ogni 12 mesi 5° anno > 5° anno Ogni 12 mesi Ogni 12 mesi A 45 gg Ogni 3 mesi Visita ginecologica X X X X X E.O. generale X X X X X Colposcopia X X X X X X X X Pap-Test X Rx torace X X X X X X RMN addome-pelvi* Urinocoltura (+ ev. Abg) X X X X CA125 X X X SCC X X X Rettoscopia *TAC addome/pelvi qualora RMN controindicata X X
Scaricare