ottava lezione
disturbi dell’emostasi
classificazione dei disordini emorragici
• difetto coagulativo
• difetto piastrinico
• difetto parete vascolare
Anamnesi dei difetti emorragici
Lividi anormali
Sanguinamento anomalo da cute e ferite
Sanguinamenti nasali
Menorragia
Emartrosi
Sanguinamento dopo estrazioni dentali
Sanguinamento durante il parto
Sanguinamento durante chirurgia
Uso di farmaci
Anamnesi familiare
Sanguinamento dal cordone ombelicale
CARATTERISTICHE CLINICHE DEI DISTURBI DELL'EMOSTASI
Lividi
Sanguinamento cutaneo
Coagulopatia
Estesi e su tutta la superficie corporea
Non severo
Epistassi
Infrequente
Emorragia digestiva
Emartro
Ematuria
Sanguinamento dopo
estrazione dentaria e
chirurgia
Infrequente e con lesione anatomica
sottostante
Comune nell'emofilia severa
Comune
Ritardata di 12-24 ore rispetto alla
sollecitazione funzionale
Disturbo delle piastrine/vWD
Piccoli
Profuso
Comune spesso prolungata e
severa
Comune
Molto raro
Rara
Subito dopo la sollecitazione
funzionale
Test di screening per i disturbi dell'emostasi
Test
Esame emocromocitometrico e
striscio periferico
Conta piastrinica
APTT
PT
TT e fibrinogeno
Tempo di stillicidio
PFA-100
D-dimero
Scopo
Anemia, leucemia, CID, schistociti
Piastrinopenia
Deficit di tutti i fattori della coagulazione eccetto il VII;
utile soprattutto per fattore VIII e IX.
Deficit dei fattori I, II, V, VII e X; terapia dicumarolica
Ipofibrinogenemia e disfibrinogenemia, eparina, prodotti
di degradazione della fibrina
Interazione piastrine-parete vasale
Test di funzionalità piastrinica e screening del
Willebrand
prodotti di degradazione della fibrina
difetto coagulativo
Cause congenite
Malattia di von Willebrand;
Emofilia A (deficit fattore VIII);
Emofilia B (deficit fattore IX);
Deficit qualitativi o quantitativi del fibrinogeno;
Emofilia C (deficit fattore XI);
Deficit di fattore VII;
Deficit di fattore XII, V, XIII, X e II;
Deficit combinati di fattori V + VIII, VII + VIII, VIII + IX.
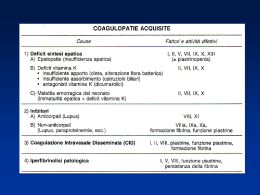
difetto coagulativo
Cause acquisite
Deficit vitamina K
Insufficienza epatica
Coagulazione intravascolare disseminata (CID)
Deficit di fibrinogeno
1. terapia con L-asparaginasi
2. morso di serpente
Anticorpi anticoagulanti circolanti (anti-fattore VIII)
1. tumori
2. LES
3. idiopatici
4. post-partum
fattore di von Willebrand Factor (vWF)
• sintetizzato nei megacariociti e nelle cellule endoteliali
(MW 230.000 kD)
• formazione di multimeri a livello plasmatico
(MW 1x106 - 10x106 kD)
.
• carrier del fattore VIII a livello plasmatico
• multimeri ad alto peso molecolare
• molto efficaci nel mediare l’adesione piastrinica
• preponderanti nelle cellule endoteliali e nel subendotelio;
Struttura vWF
vonWillebrand Disease
vonWillebrand Disease (VWD) is the most common inherited bleeding disorder, with an
estimated incidence as high as 1 in 100 to 1000.
Classification: There are three main types of VWD.
1. Type I - by far the most common form. Inheritance is autosomal
dominant. Most patients are heterozygotes.
Mild to moderate decrease in plasma VWF concentrations.
Most patients have mild disease with excessive bleeding after
surgery or trauma.
2. Type II - Rare, patients have normal levels but dysfunctional VWF,
leading to decreased VWF activity.
IIa - deficiency in high and medium molecular weight VWF due to
either inability to secrete them from the megakaryocyte or endothelial
cell or due to proteolysis as soon as they enter the circulation.
IIb - also a deficiency in high molecular weight VWF, but due to
inappropriate binding of VWF to platelets.
3. Type III - Severe decrease in plasma VWF levels. Most individuals are the
offspring of two Type I patients and therefore homozygous.
Options for therapy:
1. Cryoprecipitate (which is rich in VWF) or Factor VIII concentrates (which
contain some high molecular weight VWF)
For minor bleeding such as epistaxis, a single dose may suffice.
Perioperatively, either must be given twice a day until 48 - 72 hours
post-op
2. DDAVP (desmopressina)
Triples the plasma VWF in normal patients and in those with mild
VWD.
Not effective in Type III, because there is no VWF to begin with.
Should do trial to ensure that patient will respond before actually
using DDAVP for treatment.
Effects last 2-3 days before tachyphylaxis can occur
3. Note: Aspirin is totally contraindicated in patients with VWD because
of inhibitory effect on platelet aggegration. This would exacerbate VWD.
There is an acquired form of VWD, which occurs when antibodies inhibit VWF or when
tumors (such as lymphoid tumors) that adsorb VWF on to their surface are present.
Malattia di von Willebrandt
I.Epidemiology
A .Most common inherited bleeding disorder
B.Mild bleeding disorder (often undiagnosed)
C.Autosomal dominant disorder
II.Pathophysiology
A.Von Willebrand factor mediates platelet adhesion
B.vWF Deficiency results in mucocutaneous bleeding
III.Symptoms and Signs
A.Severe Menorrhagia (common presentation in women)
B.Postpartum Hemorrhage several days after delivery
IV.Labs
A.Lab Results vary over time in each patient
B.Partial Thromboplastin Time (PTT) prolonged
C.Bleeding Time prolonged
V.Management
A.Synthetic hormone arginine vasopressin (DDAAVP)
1.Indicated for surgery or trauma
B.Cryoprecipitate
I. Review - Factor VIII(FVIII):
Factor VIII is the precursor to Factor VIIIa, which catalyzes the activation of Factor IX to
IXa. Protein C and Protein S function to inactivate Factor VIIIa.
FVIII normally circulates in the plasma bound to vonWillebrand Factor. This association
has several functions including protecting FVIII from proteolysis, enhancing FVIII
synthesis, and concentrating FVIII at the site of active hemostasis.
FVIII is a dimer with A,B,and C domains on each monomer. When activated, it becomes a
trimer which has lost most of the B domain.
II. Hemophilia A
A. Inheritance is X-linked recessive.
B. Incidence is 1 in 5000 live male births.
C. Mechanism - quantitative deficiency in the synthesis of FVIII
D. Clinical features
1. Suspect hemophilia in any male who has history of extensive bleeding after
trauma or spontaneous bleeding into joints and muscles.
2. Excessive bleeding at time of circumcision
3. Severity of bleeding depends on level of FVIII
Severe hemophilia - FVIII < 1% - at risk for spontaneous hemorrhages and soft
tissue bleeds.
Moderate hemophilia - FVIII 1-5%
Mild hemophilia - FVIII > 5% - little risk for spontaneous bleeds but may bleed
excessively following surgery or trauma.
E. Lab abnormalities
1. Prolongation of aPTT (variable with degree of hemophilia)
2. Decreased FVIII acitvity
3. Normal vonWillebrand Factor and Ristocetin cofactor
4. Normal bleeding time
F. Management
1. Avoid aspirin
2. Mild hemophilia - prophylaxis with desmopressin (DDAVP) before dental
procedures or minor surgery. Desmopressin induces release of stored FVIII and
vonWillebrand Factor. If the increase in FVIII activity is not sufficient, administer
FVIII concentrates. Antifibrinolytics such as aminocaproic acid may be helpful. For
major surgery, give FVIII concentrates perioperatively.
3. Moderate and Severe hemophilia - give FVIII concentrate at the earliest sign
of bleeding. Desmopressin will not be helpful because these patients do not have
enough stored FVIII.
4. The amount of FVIII to give depends on the severity of the bleed.
Major bleeding or surgery - 50-100% replacement
Hemarthroses - 25% replacement
For major bleeding, treat for 10 days.
To prevent hemarthosis induced joint destruction, some propose prophylactic FVIII
concentrates three times a week to maintain trough levels of FVIII activity between
1-3%.
Concentrati di fattori della coagulazione disponibili in Italia
Tabella. Concentrati di FVIII attualmente disponibili
Prodotto
(Distributore)
Purificazione
Inattivazione
virale
Haemate P
(Centeon)
Precipitazione multipla
Pasteurizzazione 10 ore a 60°C
Emoclot D.I. (ISI)
Fanhdi
(Grifols)
Alphanate (Alpha)
Immunate (Baxter)
Beriate P (Centeon)
Hemofil M (Baxter)
Cromatografia scambio ionico Solv./det. + 30 min. a 100°C
Plasma-derivati:
Ricombinanti:
Cromatografia affinità eparina Solv./det. + 72 ore a 80°C
Cromatografia affinità eparina Solv./det. + 72 ore a 80°C
Cromatografia scambio ionico Det. + vapore 10 h 60°, 1h 80°
Cromatografia scambio ionico Pasteurizzazione 10 ore a 60° C
Cromatografia immunoaff. Solv./det.
Cromatografia immunoaff.
Kogenate (Bayer)
Helixate (Centeon)
Cromatografia immunoaff.
Recombinate (Baxter)
Cromatografia immunoaff.
Refacto (Wyeth Lederle) Cromatografia immunoaff.
8 ore a 40°C
8 ore a 40°C
-Solv./det
VIII: C
%
Emartri,
ematomi
minori
Emartri,
ematomi
maggiori
Interventi
chirurgici
DOSE DURATA
U/Kg
gg
15-30
8-15
1-2
20-30
10-25
1-6
80-100
40-70
3-10
Il fattore VIII ricombinante è disponibile per
• Prima priorità - Pazienti mai precedentemente trattati
• Seconda priorità - Pazienti HCV e HIV negativi
• Terza priorità - Pazienti con infezione da HIV
• Quarta priorità - Pazienti con infezione da HCV
III. Factor VIII Inhibitors
A. Acquired, not inherited
B. They are antibodies that neutralize FVIII and can either be alloantibodies
against exogenous FVIII or autoantibodies.
C. They are a serious complication of FVIII treatment in the severe hemophiliac
population where the incidence of developing an inhibitor is 15-20%..
D. Inhibitors can also occur in non-hemophiliac patients in the setting of:
1. Idiopathic conditions - i.e. otherwise normal elderly individuals.
2. Autoimmune disorders such as systemic lupus erythematosus, rheumatoid
arthritis, etc.
3. Malignancies such as lymphoproliferative disorders, lymphomas, and solid
tumors.
4. Drug reactions to penicillin, chloramphenicol, phenytoin, etc.
5. Pregnancy and the postpartum state
E. Risk factors for development of antibodies
1. Severity of hemophilia: Patients with severe hemophilia are at much higher risk
because they are exposed to more FVIII therapy.
2. Age of the patient and degree of FVIII exposure: 15 -20% of heavily treated
hemophiliac children develop antibodies by the age of 20.
3. Genetic defect that results in the absence of synthesis of FVIII protein: These
patients are at greater risk for developing antibodies after treatment with FVIII
concentrates.
F. Characteristics of FVIII antibodies
1. The majority of FVIII inhibitors are IgG antibodies, more specifically of the IgG4
subclass.
2. There are two main types of antibodies
Type I antibodies - seen in classic hemophiliacs. Their inhibition of FVIII follows
linear kinetics.
Type II antibodies - these are the autoantibodies and they exhibit a more complex
pattern of inhibition.
3. Antibodies are identified by their ability to neutralize FVIII at 37 degrees Celcius
after incubation for 2-3 hours.
G. Diagnosis
1. Clinical suspicion
a. Whenever classic hemophiliacs show a decreased response to FVIII
replacement therapy, screen for FVIII inhibitor.
b. Acquired inhibitors should be suspected when non-hemophiliac patients
present with spontaneous hematomas with sudden, life-threatening bleeds.
2. Laboratory information
a. aPTT - will be prolonged and will not correct upon mixing patient's plasma
with normal plasma.
b. However, the presence of FVIII inhibitor can be confirmed by adding
inhibitor plasma with normal plasma and then assaying all the residual
clotting factors in the normal plasma.
c. Quantification of the inhibitor can be done with the Bethesda Assay
H. Characteristics of patients - Grouped as low responders or high responders
1. Low responders are patients who usually have low titers of inhibitor (less than 10
BU), which does not increase after challenge with FVIII. Therefore, these patients
do not show significant anamnestic response with subsequent FVIII treatments.
2. High responders are mostly hemophiliacs who develop a high titer of inhibitor
when challenged with FVIII.
I. Management
Low responders
a. Mild bleeding episodes - options include:
Prothrombin complexes - contain all Vitamin K-dependent clotting
factors (II, VII, IX, X, and thrombin)
Activated prothrombin complexes - contain activated clotting factors
Recombinant FVIII
The first two are bypass agents because they do not contain FVIII.
Other bypass agents include Factor VIIa and Factor IXa, which
bypass the necessity for FVIII mediated activation of FIX. However,
these two are under clinical trials currently and may be associated
with increased risk of thrombosis.
b. Severe bleeding - options include:
High dose human Factor VIII
Porcine Factor VIII (see above)
Factor VIIa or another by pass agent
High responders
a. Mild bleeding episodes - considerations include:
Avoiding FVIII products to prevent an anamnestic response, which
would preclude this treatment from being a treatment option in the
event of future bleeding episodes.
b. Severe bleeding episodes
Use human or porcine FVIII until an anamnestic response occurs,
after which a bypass agent must be used instead.
5. Alternative therapies
a. Immunosuppressive agents to control antibody production
Corticosteroids
Cyclophosphamide
Azithoprine
b. Intravenous gamma globulin - in the hope that some antibodies in this
pooled sample may be directed against the inhibitor
c. Induction of immune tolerance to suppress inhibitor formation by
administration of FVIII on a continuous basis.
Factor IX Deficiency
I. Review - Factor IX(FIX):
Factor IX is the precursor to Factor IXa, which is a Vitamin K-dependent serine protease
that catalyzes the activation of Factor X to Xa
FIX normally circulates in the plasma in an inactive form. Factor VIIIa plays a role in the
enzymatic activation of FIX to FIXa.
FIX can also be activated directly by Tissue Factor - Factor VIIa complex in the extrinsic
pathway.
FIXa is a serine protease.
II. Hemophilia B (Christmas Disease)
A. Inheritance is X-linked recessive.
B. Incidence is 1 in 30,000 live male births.
C. Mechanism - quantitative deficiency in the synthesis of FIX
D. Clinical features - identical to Hemophilia A
1. Suspect hemophilia in any male who has history of extensive bleeding after
trauma or spontaneous bleeding into joints or muscles.
2. Bleeding at time of circumcision
3. Severity of bleeding depends on level of FIX
Severe hemophilia - FIX < 1% - at risk for spontaneous hemorrhages and soft
tissue bleeds.
Moderate hemophilia - FIX 1-5%
Mild hemophilia - FIX > 5% - little risk for spontaneous bleeds but may bleed
excessively following surgery or trauma.
E. Lab abnormalities
1. Prolongation of aPTT (variable with degree of hemophilia)
2. Decreased FIX acitvity
3. Normal bleeding time and thrombin time.
Prodotto
(Distributore)
Purificazione Inattivazione
virale
Plasma-derivati Cromatografia Solv./det. + 30 min.
a 100°C
Aimafix D.I. (ISI) anionica
Cromatografia Det.+vapore 10h
Immunine (Baxter)
anionica
60°, 1h 80°
Cromatografia Solv./det. +
Alphanine (Grifols)
anionica
nanofiltrazione
Cromatografia Sodio tiocianato
Mononine (Centeon)
immunoaff.
Ultrafiltrazione
Ricombinanti Cromatografia Ultrafiltrazione
Nanofiltrazione
Benefix (Baxter)
IX:C
%
DOSE
U/Kg
DURATA
gg
Emartri, ematomi minori
8-30
8-30
1-2
Emartri, ematomi maggiori
15-50
15-50
1-6
Interventi chirurgici
40-100
40-100
3-10
F. Management
1. Avoid aspirin
2. Mild hemophilia - Antifibrinolytics such as aminocaproic acid may be helpful. For
major surgery, give FIX concentrates perioperatively. For surgery, the FIX activity
should be maintained between 30-60%. Higher levels may lead to thrombosis (see
below).
3. Moderate and Severe hemophilia - give FIX concentrate at the earliest sign of
bleeding.
4. Available forms of FIX
FIX concentrates
Prothrombin complex - contains the inactive Vitamin K-dependent clotting factors
Activated prothrombin complex - contains the active Vitamin K-dependent clotting
factors.
Fresh frozen plasma - contains FIX. Limited by the fact that unless exchange
transfusion is done, sufficient FFP cannot be given to patients with severe
hemophilia to raise FIX levels sufficiently in order to prevent or control bleeding
episodes.
CID (Coagulazione Intravascolare Disseminata)
A. Infection
E. Immunologic Disorders
B. Neoplastic disease
1. Immune complex disorders
1. Mucin-Secreting adenocarcinoma
2. Allograft rejection
2. Promyelocytic Leukemia
3. Incompatible blood transfusion
3. Prostate Cancer
4. Anaphylaxis
4. Lung Cancer
F. Metabolic
C. Tissue Damage
1. Diabetic Ketoacidosis
1. Trauma
G. Miscellaneous
2. Surgery (e.g. Prostate Surgery)
1. Shock
3. Heat Stroke
2. Snake Bite
4. Burn injury
3. Cyanotic Congenital Heart Disease
5. Dissecting aneurysm
4. Fat embolism
D. Obstetrical Complication
6. Cavernous Hemangioma
1. Abruptio Placentae
2. Amniotic Fluid Embolism
3. Retained fetal products
4. Eclampsia
esami di laboratorio della CID:
• PT allungato
• aPTT allungato
• d-dimero aumentato
• fibrinogeno ridotto
• piastrine ridotte
terapia della CID:
• rimuovere la causa
• AT-III
• plasma
• eparina
• anti-fibrinolitici
difetto parete vascolare
• aumentata fragilita’ vascolare
• porpora non-trombocitopenica
• cause:
A. Eta’ (porpora senile)
B. Farmaci
C. Deficit vitamina C
D. Infezioni
D. Malattie del collagene (vasculiti)
E. Paraproteinemia (amiloidosi, crioglobulinemia)
F. Telangectasia emorragica ereditaria
G. deposizione da immunocomplessi
malattia da siero
porpora di Henoch-Schonlein
Causes of thrombocytosis
Secondary or reactive thrombocytosis
Infection (acute and chronic)
Inflammatory disorders (eg Kawasaki's disease)
Chronic iron deficiency
Acute or chronic blood loss
Tissue damage from trauma or surgery
Medicines (steroids)
Splenectomy
Malignancy (Hodgkin's disease, solid tumours)
Rebound from chemotherapy
Primary thrombocytosis
Essential thrombocytosis
Chronic myeloid leukaemia
Polycythaemia vera
Myelofibrosis
Myelodysplastic syndromes
Trombocitopenia:
1) aumentata distruzione/aumentato consumo
2) ridotta produzione
3) pseudopiastrinopenia (conteggio EDTA, citrato, eparina)
trombocitopenia da aumentata distruzione
Immune-Mediated
Non-immune Mediated
1. Drug induced Thrombocytopenia (heparin)
2. Idiopathic Thrombocytopenic Purpura (ITP)
3. Vasculitis
4. Autoimmune Hemolytic Anemia
5. Chronic Lymphocytic Leukemia (CLL)
6. Systemic Lupus Erythematosus (SLE)
7. Lymphoma
8. Human Immunodeficiency Virus (HIV)
9. Cytomegalovirus (CMV)
10. Herpes Virus infection
1. Prosthetic heart valves
2. Thrombotic Thrombocytopenic Purpura (TTP)
3. Sepsis
4. Disseminated Intravascular Coagulation (DIC)
5. Hemolytic Uremic Syndrome (HUS)
6. Hemorrhage with extensive transfusion
7. Hypersplenism
trombocitopenia da ridotta produzione
1.Infiltrative process
Acquired
2.Suppression of Megakaryocytes
Hereditary
a. Leukemia
b. Histiocytosis
c. Lymphoma
d. Myelofibrosis
e. Storage disease
f. Neuroblastoma
g. Granulomatosis
h. Osteopetrosis
a. Alcoholism
b. Megaloblastic anemia
b. Radiation
c. Infection
d. Medications
e. Aplastic Anemia
1. Thrombocytopenia-absent radii (TAR syndrome)
2. Fanconi's Anemia
3. Wiskott-Aldrich syndrome (x-linked condition)
4. May-Hegglin anomaly
5. Congenital amegakaryocytic thrombocytopenia
alterazioni qualitative delle piastrine
Hereditary defects
• Defects of platelet adhesion
Bernard-Soulier disease ("giant platelets syndrome")
Von Willebrand's disease
• Defects of platelet secretion
Storage-pool disease.
Gray-platelet disease:
• Defects of platelet aggregation
Thrombasthenia (Glanzmann's disease)
Acquired defects:
• NSAID
aspirin (permanently inhibits cyclooxygenase)
non-aspirin NSAID (temporarily block cyclo-oxygenase)
trombosi
arteriosa
venosa
piastrine
aterosclerosi
fattori della coagulazione
ipercoagulabilita’
Stato trombofilico
• trombosi in eta’ giovanile (< 50 anni)
• familiarita’ per trombosi
• trombosi ricorrenti
• trombosi in sedi insolite
• gravidanze ripetutamente complicate da aborti
cause frequenti
primari (ereditari)
cause meno frequenti
secondari
Factor V Leiden Deficiency (APCR)
Prothrombin 20210
Hyperhomocysteinemia
Antithrombin III deficiency
Protein C Deficiency
Protein S Deficiency
Factor VIII Increased
Fibrinolysis
Dysfibrinogenemia
Pregnancy
Oral Contraceptives
Estrogens
Estrogen Replacement therapy
Surgery
tamoxifen
Trauma
Infection or Sepsis
Malignancy
Myeloproliferative disorder
Hyperlipidemia
Homocystinuria
Lupus Inhibitor (LAC)
Antiphospholipid Antibodies (ACL)
Nephrotic Syndrome
Activated Protein C Resistance (Factor V Leiden)
A. Inheritance - autosomal dominant
B. Epidemiology
1. Estimated to occur in 25-40% of patients with family history of thrombosis.
2. It also occurs in 3-5% of apparently normal individuals.
C. Mechanism: the arginine at position 506 is substituted with glutamine.
This renders FVa resistant to cleavage by APC.
D. Clinical Features
1. Mean onset of DVT: 44 in heterozygotes, 31 in homozygotes;
2. Increased risk of venous thrombosis and pulmonary embolism. Venous thrombosis
occurs most frequently in the deep veins of the lower extremities.
3.Thrombosis may be precipitated by surgery, trauma, pregnancy, OCP use, or infection.
4. Arterial thrombosis is not increased.
Thrombophilic Status
Relative Risk of DVT
Normal
Oral contraceptive (OCP) use
Factor V Leiden, heterozygous
Factor V Leiden, heterozygous + OCP
Factor V Leiden, homozygous
Factor V Leiden, homozygous + OCP
Prothrombin Gene Mutation, heterozygous
Prothrombin Gene Mutation, homozygous
Prothrombin Gene Mutation, heterozygous + OCP
Protein C deficiency, heterozygous
Protein C deficiency, homozygous
Protein S deficiency, heterozygous
Protein S deficiency, homozygous
Antithrombin deficiency, heterozygous
Antithrombin deficiency, homozygous
Hyperhomocysteinemia
Hyperhomocyst + Factor V Leiden, heterozygous
1
4
5 to 7
30 to 35
80
>100
3
???
16
7
Severe DVT at birth
6
Severe DVT at birth
5
lethal prior to birth
2 to 4
20
Management
A.High Risk Indications for life-long Anticoagulation
1.Two or more spontaneous thrombotic events
2.One spontaneous life-threatening event (PE)
3.One spontaneous event with high risk cause
a. Antiphospholipid Syndrome
b. Antithrombin III deficiency
c. More than one inherited abnormality
B.Moderate Risk Indications for event-based prophylaxis
1.One event with known provocative stimulus
Farmaci per il controllo dell’emostasi
procoagulanti
piastrine
plasma
derivati del plasma
fattori ricombinanti
inibitori della fibrinolisi
vitamina K
anticoagulanti
eparina
dicumarolici
attivatori del plaminogeno
anti-aggreganti piastrinici
Scaricare