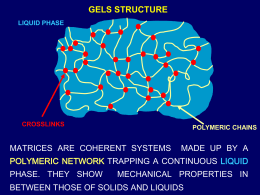
MECCANISMI DI RILASCIO DI FARMACI DA MATRICI POLIMERICHE MARIO GRASSI UNIVERSITA’ di TRIESTE Dipartimento di Ingegneria Chimica e dei Materiali STRUTTURA DELLE MATRICI POLIMERICHE LIQUID PHASE CROSSLINKS POLYMERIC CHAINS MATRICES ARE COHERENT SYSTEMS MADE UP BY A POLYMERIC NETWORK TRAPPING A CONTINUOUS LIQUID PHASE. THEY SHOW MECHANICAL PROPERTIES IN BETWEEN THOSE OF SOLIDS AND LIQUIDS 20 mm 0.2 mm Schneider et al. J. American Chemical Society, 2002. (a) Laser scanning confocal microscopy. Green regions are fluorescently stained self-assembled peptide, and black regions are water-filled pores and channels. (b) CryoTEM. Dark structures are selfassembled peptide scaffold, while lighter gray areas are composed of vitrified water. PHYSICAL CROSSLINKS (weak) ENTANGLEMENTS (TOPOLOGICAL CONSTRAINS) CONNECTING DISORDERED ZONES Van der Walls, dipole-dipole, hydrogen bonding, Coulombic hydrophobic interactions ORDERED ZONES POLYSACCARIDES (GLUCANS, XANTHAN) PHYSICAL CROSSLINKS (strong) Ca++ Ca++ Ca++ Ca++ Ca++ Ca++ EGGS BOX STRUCTURE Ca + Ca2++ O INTERACTION BETWEEN THE BIVALENT ION AND GULURONIC UNIT O OH OH HO O O OH O OH O O ALGINATES OH CHEMICAL CROSSLINKS (strong: covalent bond) SCLEROGLUCAN CROSSLINKED WITH BORAX T. Coviello et al., Int. J. Biol. Macromolecules, 32 (2003) 83 GEL SUPERPOROSI a) Monomer dilution e) Oxidant a) Monomer dilution b) Neutralization f) Reductant b) Neutralization c) Crosslinker g) Bicarbonate c) Crosslinker f) Reductant d) Foaming aid g) Bicarbonate d) Foaming aid and stabilizer SPH e) Oxidant thermal initiator SAP Figure 6.2. Schematic representation of steps involved in the production of Super porous hydrogels (SPH) and Super absorbent polymers (SPA) (with permission from ref.[46]). MATRICI LIPOFILE: Topologia SOLVENTE DELL’AMBIENTE DI RILASCIO ECCIPIENTE LIPOFILO ECCIPIENTE IDROFILO DRUG COMPRESSE POLIMERO Farmaco + Eccipienti + SISTEMA POROSO SISTEMI INORGANICI POROSI: ZEOLITI MCM-41 transmission electron micrograph. Hexagonally arranged 4.0 nm sized pores can be detected Hexagonal Array Surfactant Micelles Micellar Rod Silicate a Silicate b Calcination MCM-41 Two possible pathways for the formation of MCM-41: (a) liquid-crystal initiated b) silicate-initiated POROSITA’ FARMACO 2*RD RP CATENE POLIMERICHE RD/RP 0.01 MEZZO POROSO Il moto del farmaco avviene nel fluido di rilascio che riempe i canali le cui pareti sono costituite dal polimero ZONA 0.1 MEZZO CONTINUO INTERMEDIA Il moto del farmaco avviene tra le maglie del reticolo polimerico contenenti anche le molecole del fluido di rilascio DIFFUSIONE R=0 DRUG R = Rp De = Dw *e/t TORTUOSITA’ Lc/Rp POROSITA’ Vv/VT FISICA DEL PROBLEMA: IL RILASCIO farmaco solvente Fronte di swelling6 Matrice secca: Fronte di in questa condizione il principio attivo non erosione6 può diffondere nel reticolo polimerico TRE DIVERSI FRONTI: UNA COMODA SEMPLIFICAZIONE Fronte di swelling Fronte di diffusione Fronte di erosione Matrice rigonfiata DRUG Matrice non rigonfiata SOLVENTE SWELLING STATE DRY STATE Driving force DmH2O Chem. Pot. Dif. Counter force K(T) Chem. Pot. Dif. Crosslink density Polymeric chains pass from one equilibrium state to another one due to the incoming solvent The time required to get the new equilibrium condition is the so called relaxation time tp depending on local solvent concentration and temperature tp = polymeric chain relaxation time ts = solvent characteristic diffusion time ( L /Ds) 2 tp << ts FICK law holds (constant diffusion coefficient) tp ts tp >> ts FICK law does not hold FICK law holds (concentration dependent diffusion coefficient) D F C L C0 h C0 h FICK LAW CL F instantaneously modifies with the concentration gradient D(t ) CL C0 F h C0 h FICK LAW CL F does not instantaneously modify with the concentration gradient: F is also time dependent (D=D(t)) 100 Mt+ SOLVENT UPTAKE 110 100 90 80 70 60 50 40 30 20 10 0 Rd = 10 0 Mt De = 0 De = 1 De = 10 De = infinito 0.2 0.4 Mt 0.5 Kt M Legge di FICK 0.6 0.8 1 1.2 1.4 1.6 (t+)0.5 De = cost * t 1.8 DRUG RELEASE 100 Mt+ 100 10 De = 0 De = 1 De = 10 De = infinito Legge di FICK 1 0.1 1 td + 10 100 De = cost * t Agente rigonfiante Matrice Farmaco Dissoluzione e ricristallizazione Diffusione del farmaco Ricristallizzazione ed accumulo nell’ambiente di rilascio RICRISTALLIZZAZIONE7 T, P, SA T, P, SB POLIMORFO A SOLVENTE POLIMORFO B SOLVENTE FORMA IDRATA SOLVENTE CRISTALLO + FORMA ANIDRA + AMORFO + SA >> SB EROSION PHYSICAL REASONS 1. hydrodynamic CHEMICAL REASONS 1. Hydrolysis 2. Chemical reaction 3. Enzyme attack EROSION SURFACE EROSION 1. CHEMICAL 2. PHYSICAL BULK EROSION 1. CHEMICAL SURFACE EROSION BULK EROSION SURFACE EROSION: MECHANISM Semicrystalline polymers Amorphous polymers Disentanglements: REPTATION RELEASE FROM ERODING SYSTEM MATRICI LIPOFILE: rilascio SOLVENTE DELL’AMBIENTE DI RILASCIO ECCIPIENTE LIPOFILO ECCIPIENTE IDROFILO DRUG DISSOLUZIONE DIFFUSIONE IMPRINTED POLYMERS MOLECULAR IMPRINTING I I I COMPLEX FORMATION I I I CROSSLINKING I = initiator = template = functional monomers = crosslinking monomers WASHING IMPRINTED POLYMERS: CHARACTERISTICS Binding affinity: a measure of how well the template molecule is attracted to the binding site Selectivity : the ability to differentiate between the template and other molecules Binding capacity : the maximum amount of template bound per mass or volume of polymer BINDING AFFINITY M T MT kf Macromolecular sites concentration kr Template concentration Rf k f M T Forward reaction (binding) Rr kr MT Backward reaction (un-binding) kf 1 MT Ka Association k r K d M T constant SELECTIVITY a = Ka1/Ka2 1≤a≤8 EXAMPLE : SWELLING CONTROL A A A A A A P = PROTEIN A A A = DRUG A =ANALYTE NETWORK SWELLING: DRUG CAN BE RELEASED EXAMPLE 2: TARGETED DELIVERY TISSUES OR CELLULAR LINING HYDROGEL R DRUG IMPRINTED FILM R CELLULAR RECEPTOR 1) SWELLING 5) DIFFUSION 3) DISSOLUTION Solid drug 2) EROSION 4) RE-CRYSTALLIZATION Polymeric network 6) DRUG-POLYMER INTERACTION 7) DRUG DISTRIBUTION 8) MATRIX GEOMETRY 9) MATRICES POLYDISPERSION CARICAMENTO: SOLVENT SWELLING Farmaco Polvere polimerica 2a soluzione Allontanamento del solvente 1a soluzione Farmaco incorporato in forma cristallina e amorfa CARICAMENTO: FLUIDI SUPERCRITICI I fluidi supercritici hanno una densità comparabile a quella dei liquidi (alto potere solvente) ed una viscosità comparabile con quella dei gas (alto coefficiente di diffusione). CARICAMENTO Farmaco + CO2 ESTRAZIONE CO2 P.p. caricata per solvent swelling Polvere polimerica Farmaco incorporato in forma cristallina e amorfa Solvente solubilizzato in CO2 CARICAMENTO: COMACINAZIONE + Farmaco Polvere polimerica Mulino: energia meccanica Farmaco incorporato in forma cristallina e amorfa polimero farmaco Mezzi macinanti BIBLIOGRAFIA 1) Pharmacos 4, Eudralex Collection, Medicinal Products for Human Use: Guidelines. Volume 3C, p. 234 (internet site: http://pharmacos.eudra.org/F2/eudralex/vol3/home.htm). 2) Israel G. in Modelli Matematici nelle Scienze Biologiche, a cura di P. Freguglia, Edizioni Quattro Venti, Urbino, pag. 134 (1998). 3) Lapasin R, Pricl S, Rheology of Industrial Polysaccharides; Theory and Applications, Chapman and Hall, London, 1995. 4) Coviello T, Grassi M, Rambone G, Santucci E, a Carafa M , Murtas E, Riccieri F M, Franco Alhaique F. Novel hydrogel system from scleroglucan: synthesis and characterization J. Contr. Rel. 60, 367–378, 1999. 5) A. Kydonieus (Ed.), Treatise on Controlled Drug Delivery, Marcel Dekker, New York, 1992, pp. 54-55. 6) Colombo, P. 1993. Swelling-controlled release in hydrogel matrices for oral route. Adv. Drug. Dev. Rev., 11, 37 – 57 7) Nogami H, Nagai T, Youtsunagi T. Dissolution phenomena of organic medicinals involving simultaneous phase changes. Chem. Pharm. Bull. 17(3), 499-509, 1969. 8) Lee P I, Initial concentration distribution as a mechanism for regulating drug release from diffusion controlled and surface erosion controlled matrix systems, J. Contr. Rel. 4, 1–7, 1986. 9) Grassi M, Colombo I, Lapasin R. Drug release from an ensemble of swellable crosslinked polymer particles. J. Contr. Rel. 68, 97-113, 2000.
Scaricare