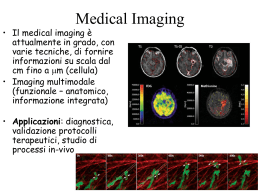
A T E P E Presented by Piero A. SALVADORI L A collaborative research project of CNR Institute of Clinical Physiology - Pisa University of Pisa - Department of Pharmaceutical Sciences CNR Institute of Biomolecular Chemistry - Pozzuoli II S Positron Emission Tomography and Amyotrophic Lateral Sclerosis: Study of Cannabinoid subtype 2 receptor expression in ALS experimental model L II A T E P • Why a proposal based on Positron Emission Tomography • Mining unexploited resources, merging hightech technologies & skills • Workplan • Project update S Outline: • Scientific background of the project & aims L II E P Aim • Development of PET tracers that selectively bind to the CB2R receptor and highlight their upregulation in neuroinflammatory conditions. • In vivo assessment of candidates by using an experimental model of ALS (mSOD1G93A transgenic mice) • Understanding whether PET in association with CB2-ligands might be considered for translational study to qualify as imaging biomarker for ALS in humans II S Background • Evidence exists that ALS involves neuroinflammatory events. • Cannabinoid type 2 Receptors (CB2R) are expressed in immune cells and tissues but are weakly present in healthy CNS. However, in neuroinflammatory conditions CB2R (but not CB1R) are upregulated in the activated microglia (MG). • Activation of MG seems to precede symptoms onset in experimental models of ALS and MG has been postulated to be involved in early motor neuron degeneration. • PET allows the quantitation of the regional distribution of radiolabelled tracers in vivo, dynamically and under real tracer conditions. A T L Scientific background of the project & aims A T P PET monitoring of response to therapy PET/FDG as biomarker Courtesy of Peter MacCallum Cancer Institute Shankar LK, et al., J.Nucl.Med. 2006; 47: 1059-66 S E II L Why a proposal based on Positron Emission Tomography E II P CT MRI MRS SPET PET BIOIMAGES anatomia funzione biochimica biochemistry US + + - CT ++ +/- MRI + + +/- MRS SPET + + +/- Damage progression Molecular imaging Highlight an interaction occurring at the molecular level while retaining its regional information PET + ++ anatomy function PET/CT ++ + ++ S US A T L Why a proposal based on Positron Emission Tomography A T P activity PET DYNAMIC IMAGING Injection of labelled drug In vivo pharmacokinetic with PET/CT time …. Set of images (2D) from Volume of Interest (3D) t Frame 1 (t=t0) Frame 2 (t=t2) Frame n (t=tn) t t S E II L Why a proposal based on Positron Emission Tomography E II P Lead Molecule Discovery 500 - 3000 Patients Product launch Clinical trials Drug discovery Target ID 100 - 500 Patients Preclinical Research Phase I Animal research investments Phase II Phase III Product Licencing Industrial Production Marketing Commercialisation and Return on investments S Drug Development & Research flowchart 20 - 100 Volunteers A T L Why a proposal based on Positron Emission Tomography QuickTime™ e un decompressore sono necessari per visualizzare quest'immagine. CNR Institute of Biomolecular Chemistry - Pozzuoli Dr. V Di Marzo Structure-activity relationships & molecular pharmacology Characterisation & development of bioactive molecules Biomasses & biologically active substrates E P Radiopharmaceutical Chemistry & Imaging Biomarker development Advanced biomedical technologies In vivo/ex vivo imaging & pharmacology University of Pisa Department of Pharmaceutical Sciences Prof. C. Manera Computational Chemistry New scaffolds & Analogue modification Customised synthesis S CNR Institute of Clinical Physiology - Pisa Dr. P.A. Salvadori II L Mining unexploited resources, merging hightech resources & skills A T A T P Unsatisfactory Biochemistry Lead molecules, reference standard & chemical precursors QuickTime™ e un decompressore sono necessari per visualizzare quest'immagine. Biochemical characterisation of lead compounds Precursor design optimisation, test labelling, purification & formulation In vivo microPET & microCT, dynamic imaging & biodistribution Candidate selection by in vivo imaging & proof-of-principle demonstration Testing in disease model QuickTime™ e un decompressore sono necessari per visualizzare quest'immagine. Tissue hystology & receptor analysis Unsatisfactory Radiochemistry or Biology S E II L Workplan E P 18F II N O R1 A suitable "cold" precursor should be designed so that the expected active compound can be prepared within the constraints of labelling reaction (short time and high specific-activity) • • • S R2 X A T L Project update General structure based on aromatic fused rings Introduction of fluorine-18 label in different positions R1, R2 and other structure modifiers to modulate SAR compound Ki (CB1) Ki (CB2) CB1/CB2 CB 83 >10000 370 > 27 CB 102 500 51.8 9.6 CB 92 9.6 0.7 14 CB 91 200 0.9 222 VL 22 11.6 0.2 48 AF 4 40 7.9 5 Warning: Parent "cold" precursor should also be tested for biological activity as it may compete with the tracer for the binding site High specific activity: the mass associated to the radioactivity of tracer is negligible (<< µmol) from the point of view of toxicity and macroscopic effect, E II S P Radiochemical Radionuclide production A T L Project update • Development of labelling chemistry • Select precursor • Test labelling (yield, time scale, bulk volume, impurities & toxicity) • Synthesis & purification (radiation protection, radiochemical purity, specific activity) Radiopharmaceutical Radiochemistry Image acquisition Microfluidic radiolabelling platform Different routes for 18F "activation: counter ions, criptands, phase-transfer agents, .. Solvents, temperature, time of reaction vary with substrates and labelling chemistry <18F> n OTs CH3CN TsO R1 R2 n OTs 18 F R1 n = 2,3 R2 R3 Cl N R3 <18F> DMSO 18 N F R R= morpholin (CB26), phenyl (C41) N N R R= morpholin (CB83), phenyl (C102) A T P Examples of TAC on µCT identified organs S E II L Project update QuickTime™ and a PNG decompressor are needed to see this picture. QuickTime™ and a YUV420 codec decompressor are needed to see this picture. HIGH-RESOLUTION µCT and µPET CD56 healthy mouse (wild type) microPET/microCT image fusion A T P Lead molecules, reference standard & chemical precursors S E II L Project update Project clock START APRIL 2011 Biochemical characterisation of lead compounds ACTUALLY ON-GOING MID TERM Precursor design optimisation, test labelling, purification & formulation In vivo microPET & microCT, dynamic imaging & biodistribution END APRIL 2012 QuickTime™ e un decompressore sono necessari per visualizzare quest'immagine. Candidate selection by in vivo imaging & proof-of-principle demonstration Testing in disease model Control group (CD1 mice) 60-70 dd old (young healthy) 120-140 dd old (old healthy) Disease developing group (mSOD1 mice) Tissue hystology & receptor analysis 60-70 dd old (young asymptomatic) 90-100 dd old (symptoms onset) 120-130 dd old (serious symptoms) A T E E P We are grateful to AriSLA for supporting our research Thank you for your kind attention S L II L II
Scaricare