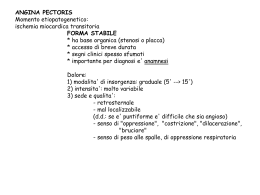
Cardiopatia Ischemica • Ischemia is characterized by an imbalance between myocardial oxygen supply and demand. In some situations this imbalance is caused by a reduction of blood flow and oxygen supply secondary to increased coronary vascular tone, intracoronary platelet aggregation, or thrombus formation. • This condition, termed supply ischemia or low-flow ischemia, is responsible for myocardial infarction and most episodes of unstable angina. • In other instances, usually in the presence of chronic coronary obstruction, exercise, tachycardia, or emotion leads to an increase in coronary blood flow that is insufficient to meet the rise in myocardial oxygen demand. This condition is termed demand ischemia or high-flow ischemia. It is responsible for many episodes of chronic stable angina. • Typically, myocardial ischemia results from both an increase in oxygen demand and a reduction in myocardial oxygen supply. For example, although exercise leads to an overall increase in coronary blood flow, most of the additional flow is distributed toward the subepicardium, whereas subendocardial blood flow may even drop below its resting level. The ischemia of the subendocardium is then caused by both an increase in myocardial oxygen demand and a reduction in regional blood flow. • Low-flow ischemia, in contrast to high-flow ischemia or hypoxia, is characterized not only by oxygen deprivation but also by inadequate removal of metabolites consequent to reduced perfusion and by loss of vascular turgor. Coronary flow and coronary perfusion pressure augment left ventricular systolic performance (Gregg effect) and reduce left ventricular diastolic distensibility (Salisbury effect). Buildup of tissue metabolites, especially inorganic phosphate, reduces calcium sensitivity of myofilaments, thereby diminishing contractility. Accordingly, in patients with low-flow ischemia, left ventricular systolic performance is lower and left ventricular diastolic distensibility greater than when the same patients were exposed to high-flow ischemia or hypoxia. • Myocardial ischemia may be manifest as anginal discomfort, breathlessness, deviation of the ST segment on the electrocardiogram, reduced uptake of a tracer substance in myocardial perfusion images, or regional or global impairment of ventricular function. Determinanti del consumo di O2 Regolazione del flusso coronarico Concetti generali • Il flusso coronarico è prevalentemente diastolico • I determinanti del flusso sono il gradiente pressorio e le resistenze coronariche • Il gradiente è dato dalla differenza tra pressione aortica (in diastole) e pressione diastolica in ventricolo sinistro • Esiste una pressione critica di chiusura Flusso coronarico totale (ml/m’) 100 200 300 Autoregolazione coronarica Flusso massimo Flusso a riposo 0 Riserva coronarica 25 50 75 100 125 150 Pressione arteriosa (mm Hg) Autoregolazione coronarica Flusso massimo Ipertensione Flusso coronarico totale (ml/m’) 100 200 300 Flusso x 100 g di Ventricolo normale Flusso x 100 g di Ventricolo ipertrofico Riserva coronarica 0 Flusso a riposo 50 75 100 125 150 175 Pressione arteriosa (mm Hg) 200 MECHANISMS OF AUTOREGULATION • NITRIC OXIDE. – Evidence suggests a role for NO in coronary autoregulation. Inhibition of NO raises the lower autoregulatory threshold by about 15 mm Hg. The involvement of NO may be related to the ability of the endothelium to sense changes in perfusion pressure through pressure-sensitive ion channels. • MYOGENIC CONTROL. – Arteriolar smooth muscle reacts to increased intraluminal pressure by contracting. The consequent augmentation of resistance tends to return blood flow toward normal despite the higher perfusion pressure. This regulatory mechanism, referred to as myogenic control, is an important mechanism in some vascular beds. Their contribution to autoregulation is relatively small. • NITRIC OXIDE (NO). – This substance increases blood flow during metabolic stimuli. NO production is augmented in response to metabolic stimuli by at least two mechanisms. Hypoxia is a stimulus to release of NO from the endothelium. Furthermore, NO is a principal mediator of flow-mediated dilation. Although hypoxia may initiate hyperemia, flow-mediated dilation sustains and amplifies it. • OTHER METABOLIC MEDIATORS. – Inhibition of the synthesis of vasodilator prostaglandins and inhibition of K+ATP channels also reduces metabolic vasodilation. A loss or inhibition of one mediator is compensated for by upregulation of others. Although the inhibition of K+ATP channels, adenosine, and NO individually has at most a modest effect on the increase in coronary blood flow during exercise in dogs, inhibition of all three simultaneously nearly abolishes the flow increase. Endotelio: un organo con 2 funzioni Eparina Prostaciclina Glycocalice Adenosina Glycocalice EDRF Antitrombina III Azione Antiadesione piastrinica Trombomodulina NO Adenosina Repulsione neutrofili Azione dilatante Azione Anticoagulante ENDOTHELIA L CELL Cellula endoteliale Azione Procoagulante Azione costrittrice Adesione Proadesione piastrinica Attrazione neutrofili Angiotensina II Fattore IX ICAM-1 Fattore X Fattore VIII di Von Willebrand Selectina P Endotelina Fattori di derivazione endoteliale Fattori di rilasciamento endotelio-derivati: EDRF • Ossido d’azoto (NO) • Fattore iperpolarizzante endotelio-derivato (EDHF) • Prostaciclina Fattori di contrazione endotelio-derivati: EDCF • Anione superossido • Trombossano • Endoperossidi • Endotelina Patologia della liberazione di EDRF Quando le cellule endoteliali scompaiono, vengono rimpiazzate da nuove cellule rigenerate. Tali cellule sono meno capaci di produrre EDRF. Diversi studi hanno dimostrato una tendenza localizzata ad una esagerata vasocostrizione che è una caratteristica precoce della malattia coronarica nell’uomo. Vanhoutte P, Boulanger MC: “L’endotelio: un ruolo fondamentale nella fisiopatologia cardiovascolare” 1994 L’Endotelio e il tono vascolare 1980: Furchgott e Zawadski scoprono che l’endotelio è essenziale per la vasodilatazione dell’acetilcolina ACETILCOLINA -9 -9 -8 -7 -6 -5 -8 -7 -6 -5 INTATTO DENUDATO 1986: il gruppo di Ignarro e quello di Moncada identificano nel NO (ossido di azoto) il fattore vasodilatante endotelio-dipendente Transmural Distribution of Myocardial Blood Flow Cross-section of the left ventricular wall in diastole and systole. Factors involved in the susceptibility of the subendocardium to the development of ischemia include the greater dependence of this region on diastolic perfusion and the greater degree of shortening, and therefore of energy expenditure, of this region during systole. Ischemia subendocardica • Il flusso subendocardico è leggermente maggiore del flusso subepicardico a riposo (rapporto =1,16) • Tuttavia il gradiente di perfusione è minore, e le forze compressive sono maggiori • Ne deriva che questo flusso maggiore è ottenuto per vasodilatazione arteriolare, cioè con riduzione della riserva coronarica Subendocardial ischemia • Epicardial coronary stenoses are associated with reductions in the subendocardial to subepicardial flow ratio. • Severe pressure-induced left ventricular hypertrophy, as well as heart failure with elevated left ventricular end-diastolic pressure, may also reduce the endocardial-to-epicardial flow ratio. When the markedly elevated left ventricular enddiastolic pressure in heart failure is corrected, subendocardial coronary flow reserve is restored and the endocardial-toepicardial flow ratio is normalized. • Reduction of myocardial oxygen demand, for example by beta blockers, also decreases epicardial blood flow and increases perfusion pressure and thereby flow to the ischemic subendocardial region. Effects of Coronary Stenoses • As blood traverses a stenosis, pressure (energy) is lost. Principles of fluid dynamics have been applied to estimate this pressure loss and validated in animals models as well as in patients. Although the formulas are complex, they can been simplified as follows: • where DP is the pressure drop across a stenosis in millimeters of mercury (mm Hg), Q is the flow across the stenosis in milliliters per second, and dsten is the minimal diameter of the stenosis lumen in millimeters Energy losses across a stenosis. The pressure gradient due to friction losses within the stenosis (DP) is directly proportional to blood flow (Q), whereas separation losses at the exit to the stenosis due to formation of eddies increase with blood flow squared (Q2). Separation losses predominate at high blood flows. Relation between pressure reduction across a stenosis (DP) and flow through the stenosis (Q) Relationship between resting (dashed line) and maximal coronary blood flow (solid line) and percentage of diameter stenosis Riserva coronarica • La riserva coronarica è il rapporto tra il flusso massimale (ottenibile con la vasodilatazione massimale) e il flusso a riposo • Normalmente questo rapporto è circa uguale a 4 • La riserva coronarica comincia a diminuire significativamente per stenosi di circa 60-70% Coronaria destra Coronaria sinistra OAdx Valutazione della riserva coronarica • Stimolo alla vasodilatazione massimale (adenosina, dipiridamolo) • Misurazione del flusso coronarico – Diretta: flussimetri intracoronarici – Indiretta: • Ecodoppler • Fractional flow reserve FFR myo SU DA BASALE 0.86 ADENOSINA i.c 0.84 Coronary Collateral Vessels • COLLATERAL FORMATION (ARTERIOGENESIS) – Preexisting collaterals are normally closed and nonfunctional, because no pressure gradient exists between the arteries they connect. After coronary occlusion, the distal pressure drops precipitously and preexisting collaterals open virtually instantly. The transformation of preexisting collaterals into mature collaterals is called arteriogenesis is characterized by inflammation and cellular proliferation. • MECHANISMS PROMOTING COLLATERAL GROWTH – SHEAR STRESS. • Pressure gradients across preexisting rudimentary collaterals augment blood flow velocity and shear stress. Shear stress induces widespread functional changes in the endothelium, many of which reflect new gene expression. – INFLAMMATION. Coronary Collateral Vessels (2) • ALTRI FATTORI – Gravità della stenosi – Ipossia – Altri fattori di rischio – Attività fisica • SIGNIFICATO FUNZIONALE – La capacità del circolo collaterale non supera il 50% di quella del circolo nativo (corrisponde quindi ad una stenosi del 70-80%) Conseguenze metaboliche dell’ischemia • Metabolismo anaerobico: produzione di acido lattico per glicolisi • Deplezione di ATP • Perdita di potassio Conseguenze emodinamiche dell’ischemia • Alterazioni reversibili: fino a 20-30 m’ • Cascata ischemica: – – – – Disfunzione diastolica Disfunzione sistolica Alterazioni ECG Dolore anginoso • Tempo di recupero inversamente proporzionale alla profondità dell’ischemia – Stunning – Hibernation Schematic diagram of stunned myocardium During coronary occlusion, a wall motion abnormality of the left ventricle is present in the region supplied by the occluded artery. With relief of ischemia and reestablishment of coronary blood flow, there is a persistent wall motion abnormality despite reperfusion and viable myocytes. There is then gradual improvement in function that requires hours to days for recovery. Two possible additive components of postischemic dysfunction: (1) reperfusioninduced pathology, which can be restored through the use of a therapeutic intervention such as an antioxidant or calcium-limiting agent given transiently at the time of reperfusion; and (2) ischemic pathology from which the heart is slowly recovering. These may be additive to each other and to any additional reperfusioninduced component that is not amenable to the chosen intervention. Hibernating myocardium • The term hibernating myocardium refers to the presence of impaired resting left ventricular function, owing to reduced coronary blood flow that can be restored toward normal by revascularization. • Hibernation was first noted in patients with coronary artery disease who had no evidence of ongoing ischemia yet whose left ventricular function improved after coronary artery bypass grafting. • Even akinetic segments can occasionally regain systolic contraction after revascularization. • Hibernating myocardium is present in approximately one third of patients with coronary artery disease and impaired left ventricular function. CHARACTERISTICS OF STUNNING, HIBERNATION, AND ISCHEMIA PARAMETER STUNNING HIBERNATION TRUE ISCHEMIA Myocardial mechanical function Reduced Reduced Reduced Coronary blood flow Post-ischemic: normal/high Modestly reduced or low normal; intermittent ischemia-reperfusion Most severely reduced Myocardial energy metabolism Normal or excessive Reduced or low normal; in steady state with intermittent ischemiareperfusion Reduced; increasingly severe as ischemia proceeds Duration Hours to days; late stunning over weeks Days to hours to months Minutes to hours Outcome Full spontaneous recovery Recovery if revascularized Myocyte necrosis if severe ischemia persists Proposed change in metabolic regulation of calcium Cytosolic overload of calcium in early reperfusion with damage to contractile proteins Possibly just enough glycolytic ATP to prevent contracture Insufficient glycolytic ATP to prevent ischemic contracture and irreversibility ATP = adenosine triphosphate. Modified from Opie LH: The multifarious spectrum of ischemic left ventricular dysfunction: Relevance of new ischemic syndromes. J Mol Cell Cardiol 28:2403–2414, 1966. Forme cliniche della cardiopatia ischemica • Angina da sforzo • Sindromi ischemiche acute – Con sopraslivellamento del tratto ST (infarto miocardico acuto) – Senza sopraslivellamento sel tratto ST • Angina instabile • Infarto non-Q • Cardio(mio)patia ischemica cronica Angina da sforzo Netter LE DIVERSE SEDI DEL DOLORE TORACICO 1: posizione del cuore nel torace 2: angina di petto 3: irradiazione nell’attacco card. 4: sede correlata ad emozioni e stati ansiosi Da: M Caccavale. Inervista sul cuore e dintorni, 1984. Adn Kronos, Roma ALCUNE POSSIBILI SEDI ED IRRADIAZIONI DEL DOLORE TORACICO DI ORIGINE ESOFAGEA Sleisenger MH, Fordtran JS: Trattato di gastroenterologia, Piccin Ed, Padova EMANAZIONE SIMPATICA E PARASIMPATICA DEL CUORE ED EMBRICAZIONE DELL’INNERVAZIONE CARDIACA E SPLANCNICA da: S Dalla Volta. La cardiopatia ischemica: dalla teoria alla clinica GRECO agcon: kardia: cappio, capestro, laccio cuore, bocca dello stomaco Liddel Scott. Le Monnier LATINO angor: stringimento, angoscia, pena cardiacus: di stomaco Castiglioni Mariotti. Loescher INGLESE heart burn: bruciore di stomaco Hazon. Garzanti ITALIANO cardias: angere: sbocco dell’esofago nello stomaco affliggere, angosciare DOLORE TORACICO DI TIPO ANGINOSO ED ATTRIBUZIONE DELLA SINTOMATOLOGIA ALL’ESOFAGO Pazienti Delmonico et al., 1968 Brand et al., 1977 Dart et al., 1980 Ferguson et al., 1981 Kline et al., 1981 De Meester et al., 1982 Katz et al., 1989 Schofield et al., 1989 Nevens et al., 1991 Voskuil et al., 1996 Frøbert et al., 1996 Chauhan et al., 1996 Fass et al., 1998 Ho et al., 1998 Börjesson et al., 1998 Romand et al., 1999 Netzer et al., 1999 117 43 98 72 16 50 16 52 37 28 46 32 37 80 20 43 303 % origine esofagea 10 46 17 18 31 46 31 48 50 36 25 66 62 46 35 44 54 Summary • Angina pectoris is a discomfort in the chest or adjacent areas caused by myocardial ischemia. • Anginal “equivalents” (i.e., symptoms of myocardial ischemia other than angina), such as dyspnea, faintness, fatigue, and eructations, are common, particularly in the elderly. • Nocturnal angina should raise the suspicion of sleep apnea. • Chest discomfort while walking in the cold, uphill, or after a meal is suggestive of angina. • Features suggesting the absence of angina pectoris include pleuritic pain, pain localized to the tip of one finger, pain reproduced by movement or palpation of the chest wall or arms, and constant pain lasting many hours or, alternatively, very brief episodes of pain lasting seconds. Pain radiating into the lower extremities is also a highly unusual manifestation of angina pectoris. • Typical angina pectoris is relieved within minutes by rest or by the use of nitroglycerin. Differential diagnosis of chest pain according to location where pain starts. Serious intrathoracic or subdiaphragmatic diseases are usually associated with pains that begin in the left anterior chest, left shoulder, or upper arm, the interscapular region, or the epigastrium. The scheme is not all inclusive (e.g., intercostal neuralgia occurs in locations other than the left, lower anterior chest area). Fisiopatologia • Abitualmente placca aterosclerotica stabile che crea stenosi critica • Angina da eccesso di domanda o da riduzione di offerta • Angina a soglia fissa/soglia variabile • Angina mista • Non c’è correlazione tra gravità del sintomo e gravità della malattia • L’ischemia può essere silente Factors influencing the balance between myocardial O2 requirements (left) and supply (right). Arrows indicate effects of nitrates. In relieving angina pectoris, nitrates exert favorable effects by reducing O2 requirements and increasing supply. Although a reflex increase in heart rate would tend to reduce the time for coronary flow, dilation of collaterals and enhancement of the pressure gradient for flow to occur as the left ventricular end-diastolic pressure (LVEDP) falls tend to increase coronary flow. AoP = aortic pressure; NC = no change. (From Frishman WH: Pharmacology of the nitrates in angina pectoris. Am J Cardiol 56:8I, 1985. By permission of Excerpta Medica.) Elettrocardiogramma • A riposo e in assenza di sintomi l’elettrocardiogramma può essere normale (spesso è normale) – Altre alterazioni possono dipendere dalla malattia di base • In presenza di sintomi (angina) il segno fondamentale è la lesione subendocardica, (sottoslivellamento del tratto ST) Elettrogenesi della “lesione” Acute ischemia may alter ventricular action potentials by inducing lower resting membrane potential, decreased amplitude and velocity of phase 0, and an abbreviated action potential duration (pathological early repolarization). These electrophysiological effects create a voltage gradient between ischemic and normal cells during different phases of the cardiac electrical cycle. The resulting currents of injury are reflected on the surface electrocardiogram by deviation of the ST segment Current-of-injury patterns with acute ischemia. With predominant subendocardial ischemia (A), the resultant ST vector is directed toward the inner layer of the affected ventricle and the ventricular cavity. Overlying leads therefore record ST depression. With ischemia involving the outer ventricular layer (B) (transmural or epicardial injury), the ST vector is directed outward. Overlying leads record ST elevation. Reciprocal ST depression can appear in contralateral leads. Test ergometrico (prova da sforzo) • Si esegue al cicloergometro o al tapis roulant (treadmill) • Esercizio a carichi crescenti monitorizzando frequenza cardiaca e pressione arteriosa • Cessazione dello sforzo se compaiono sintomi o alterazioni ECG • Positività: alterazioni del tratto ST (più spesso sottoslivellamento) Miocardioscintigrafia da sforzo (o da stress) Dobutamina ischemia apice laterale durante stress Treatment • 1) identification and treatment of associated diseases that can precipitate or worsen angina; • (2) reduction of coronary risk factors; • (3) application of general and nonpharmacological methods, with particular attention toward adjustments in life style; • (4) pharmacological management; • (5) revascularization by percutaneous catheterbased techniques or by coronary bypass surgery Mortalità annua x 100000 maschi Non fumatore Ex fumatore Fumatore < 15 15-25 >25 RR (>25/NF) BPCO 10 57 127 86 112 225 22,5 (11-42) Cardiopatia ischemica 572 678 892 802 892 1025 1,8 (1,6-2) Miocardiopatie 61 88 125 122 109 173 2,8 (2,1-3,8) Ipertensione 32 33 44 28 51 60 1,8 (1,2-2,9) Cerebrovasculopatie 152 158 166 121 231 235 1,5 (1,2-1,9) Neoplasia polmonare 14 58 209 105 208 355 25,4 (15-43) BMJ 1994;309:901-911 (8 October) Mortality Risk Reduction Associated With Smoking Cessation in Patients With Coronary Heart Disease. A Systematic Review Julia A. Critchley, MSc, DPhil; Simon Capewell, MD, FRCPE JAMA. 2003;290:86-97. Objective To conduct a systematic review to determine the magnitude of risk reduction achieved by smoking cessation in patients with CHD. Data Sources Nine electronic databases were searched from start of database to April 2003, supplemented by cross-checking references, contact with experts, and with large international cohort studies (identified by the Prospective Studies Collaboration). Study Selection Prospective cohort studies of patients who were diagnosed with CHD were included if they reported all-cause mortality and had at least 2 years of follow-up. Smoking status had to be measured after CHD diagnosis to ascertain quitting. Data Extraction Two reviewers independently assessed studies to determine eligibility, quality assessment of studies, and results, and independently carried out data extraction using a prepiloted, standardized form. Data Synthesis From the literature search, 665 publications were screened and 20 studies were included. Results showed a 36% reduction in crude relative risk (RR) of mortality for patients with CHD who quit compared with those who continued smoking (RR, 0.64; 95% confidence interval [CI], 0.58-0.71). Results from individual studies did not vary greatly despite many differences in patient characteristics, such as age, sex, type of CHD, and the years in which studies took place. Adjusted risk estimates did not differ substantially from crude estimates. Many studies did not adequately address quality issues, such as control of confounding, and misclassification of smoking status. However, restriction to 6 higher-quality studies had little effect on the estimate (RR, 0.71; 95% CI, 0.65-0.77). Few studies included large numbers of elderly persons, women, ethnic minorities, or patients from developing countries. Conclusions Quitting smoking is associated with a substantial reduction in risk of allcause mortality among patients with CHD. This risk reduction appears to be consistent regardless of age, sex, index cardiac event, country, and year of study commencement. Trattamento farmacologico • Nitrati (Trinitroglicerina e altri) – In particolare per risolvere l’attacco acuto (via sublinguale) • Beta-bloccanti • Calcioantagonisti Effects of nitrates in generating NO+ and stimulating guanylate cyclase to cause vasodilation. Note the role of cysteine cascade in stimulating guanylate cyclase. Previously, sulfhydryl (SH) depletion was thought to explain nitrate tolerance. Current emphasis is on the generation of peroxynitrite, which in turn inhibits the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (GMP). Note that mononitrates bypass hepatic metabolism. The mechanisms of action of the nitrates are complex. These drugs decrease cardiac demand and may increase coronary blood supply through a variety of actions. It is likely that the various mechanisms that act to provide relief to angina patients or prevent myocardial ischemia differ among individuals. LV = left ventricular; RV = right ventricular. Mechanisms of nitrate tolerance • • • • • • Depletion of Sulfhydryl Groups. Neurohormonal Activation. Plasma Volume Expansion. Downregulation of Receptors. Free Radical Generation. MANAGEMENT. – The only practical strategy to manage nitrate tolerance is to prevent it by providing a “nitrate-free” interval. INTERACTION WITH SILDENAFIL. • The combination of nitrates and sildenafil may cause serious, prolonged, and potentially lifethreatening hypotension. Nitrate therapy is an absolute contraindication to the use of sildenafil and vice versa. Patients who wish to take sildenafil should be aware of the serious nature of this adverse drug interaction and be warned about taking sildenafil within 24 hours of any nitrate preparation, including short-acting sublingual nitroglycerin tablets. Effects of beta blockade on the ischemic heart. Beta blockade has a beneficial effect on ischemic myocardium unless (1) the preload rises substantially as in left-sided heart failure or (2) vasospastic angina is present, in which case spasm may be promoted in some patients. Note the recent proposal that beta blockade diminishes exercise-induced vasoconstriction. CANDIDATES FOR USE OF BETA-BLOCKING AGENTS FOR ANGINA Ideal Candidates Prominent relationship of physical activity to attacks of angina Coexistent hypertension History of supraventricular or ventricular arrhythmias Previous myocardial infarction Left ventricular systolic dysfunction Mild to moderate heart failure symptoms (NYHA functional Class II–III) Prominent anxiety state Poor Candidates Asthma or reversible airway component in chronic lung disease patients Severe left ventricular dysfunction with severe heart failure symptoms (NYHA functional Class IV) History of severe depression Raynaud's phenomenon Symptomatic peripheral vascular disease Severe bradycardia or heart block Brittle diabetes NYHA = New York Heart Association. RECOMMENDED DRUG THERAPY (CALCIUM ANTAGONIST VS. BETA BLOCKER) IN PATIENTS WHO HAVE ANGINA IN CONJUNCTION WITH OTHER MEDICAL CONDITIONS CLINICAL CONDITION RECOMMENDED DRUG CARDIAC ARRHYTHMIA OR CONDUCTION DISTURBANCE Sinus bradycardia Nifedipine or amlodipine Sinus tachycardia (not caused by cardiac failure) Beta blocker Supraventricular tachycardia Beta blocker (verapamil) Atrioventricular block Nifedipine or amlodipine Rapid atrial fibrillation (with digitalis) Verapamil or beta blocker Ventricular arrhythmia Beta blocker LEFT VENTRICULAR DYSFUNCTION Heart failure Beta blocker MISCELLANEOUS MEDICAL CONDITIONS Systemic hypertension Beta blocker (calcium antagonist) Severe preexisting headaches Beta blocker (verapamil or diltiazem) COPD with bronchospasm or asthma Nifedipine, amlodipine, verapamil, or diltiazem Hyperthyroidism Beta blocker Raynaud's syndrome Nifedipine or amlodipine Claudication Calcium antagonist Severe depression Calcium antagonist COPD = chronic obstructive pulmonary disease. (alternatives in parentheses) TABLE 37–3. EFFECTS OF ANTIANGINAL AGENTS ON INDICES OF MYOCARDIAL OXYGEN SUPPLY AND DEMAND* BETA-ADRENOCEPTOR BLOCKERS ISA INDEX NITRATES No Yes Cardioselective CALCIUM ANTAGONISTS No Yes Nifedipine Verapamil Ditiazem 0 0 0 SUPPLY Coronary resistance Vascular tone 0 Intramyocardial diastolic tension 0 0 Coronary collateral circulation 0 0 0 0 0() 0 0 Preload 0 0 Afterload (peripheral vascular resistance) Duration of diastole () () () DEMAND Intramyocardial systolic tension 0 0 Contractility 0( ) ( ) Heart rate 0( ) 0 0( ) † ( ) ( ) † ( ( ) † ) TABLE 37–3. EFFECTS OF ANTIANGINAL AGENTS ON INDICES OF MYOCARDIAL OXYGEN SUPPLY AND DEMAND* BETA-ADRENOCEPTOR BLOCKERS ISA INDEX NITRATES No Yes Cardioselective CALCIUM ANTAGONISTS No Yes Nifedipine Verapamil Ditiazem 0 0 0 SUPPLY Coronary resistance Vascular tone 0 Intramyocardial diastolic tension 0 0 Coronary collateral circulation 0 0 0 0 0() 0 0 Preload 0 0 Afterload (peripheral vascular resistance) Duration of diastole () () () DEMAND Intramyocardial systolic tension 0 0 Contractility 0( ) ( ) Heart rate 0( ) 0 0( ) † ( ) ( ) † ( ( ) † ) * = Increase; = decrease; 0 = little or no definite effect. The number of arrows represents the relative intensity of effect. Symbols in parentheses indicate reflex-mediated effects. †Effect of calcium entry on left ventricular contractility, as assessed in the intact animal model. The net effect on left ventricular performance is variable since it is influenced by alterations in afterload, reflex cardiac stimulation, and the underlying state of the myocardium. ISA = intrinsic sympathomimetic activity. From Shub C, Vlietstra RE, McGoon MD: Selection of optimal drug therapy for the patient with angina pectoris. Mayo Clin Proc 60:539, 1985. By permission of the Mayo Foundation. Prognosi in terapia medica • La prognosi è riservata • In 15 mesi: – Mortalità: 4% – Infarto miocardico: 7% – Remissione spontanea: 11% • Importante il ruolo di: – Funzione ventricolare sinistra – Estensione dell’ischemia inducibile Graphs showing survival for medically treated CASS patients. A, Patients with one-, two-, or three-vessel disease and an ejection fraction of 50 to 100 percent stratified by the number of diseased vessels (DISVES). B, Patients with one-, two-, or three-vessel disease and an ejection fraction of 35 to 49 percent stratified by the number of diseased vessels. C, Patients with one-, two-, or three-vessel disease and an ejection fraction of 0 to 34 percent stratified by the number of diseased vessels. Indicazioni alla rivascolarizzazione • Mediante angioplastica: presenza di una o più stenosi critiche, preferibilmente senza coinvolgimento della discendente anteriore (indicazioni “quoad valetudinem”) • Mediante bypass aortocoronarico (indicazioni “quad vitam”): – Malattia del Tronco comune (>50%) – Malattia di tre vasi – Malattia di due vasi con coinvolgimento della discendente anteriore prossimale Adjusted hazard (mortality) ratios comparing coronary artery bypass grafting (CABG) and medical therapy for nine coronary anatomy severity groups (GR) according to the number of vessels diseased (VD), the presence or absence of a 95 percent proximal stenosis (95 percent), and involvement of the left anterior descending coronary artery (LAD). B, Adjusted hazard (mortality) ratios comparing CABG and percutaneous transluminal angioplasty (PTCA) for nine coronary anatomy groups according to the number of vessels diseased, the presence or absence of a 95 percent proximal stenosis, and LAD involvement. Two-year cumulative mortality rates for three treatment strategies. Significant differences were seen between revascularization and angina-guided strategies (P<.005) and between revascularization and ischemia-guided strategies (P<.05). Angina-guided and ischemia-guided strategies were not significantly different from each other (P=.34). Asymptomatic Cardiac Ischemia Pilot (ACIP) Study Two-Year Follow-up : Outcomes of Patients Randomized to Initial Strategies of Medical Therapy Versus Revascularization. Circulation, Apr 1997; 95: 2037 - 2043. Angina instabile Angina instabile / Nomenclatura • Unstable angina lies in the center of the spectrum of clinical conditions caused by myocardial ischemia. These range from chronic stable angina pectoris to the acute coronary syndromes. • The latter, in turn, consist of acute myocardial infarction (MI) associated with electrocardiographic ST segment elevation (STEMI) and unstable angina/non-ST segment elevation MI (UA/NSTEMI). • The former is most commonly caused by acute total coronary occlusion, and urgent reperfusion is the mainstay of therapy, • UA/NSTEMI is usually associated with severe coronary obstruction but not total occlusion of the culprit coronary artery. • If the myocardial ischemia that results from the coronary obstruction is long in duration and/or great in severity, myocardial necrosis occurs, and the patient is classified as having a non-Q-wave MI or, now more aptly termed, NSTEMI Definizione clinica • Unstable angina is defined as angina pectoris (or equivalent type of ischemic discomfort) with at least one of three features: – (1) it occurs at rest (or with minimal exertion) usually lasting more than 20 minutes (if not interrupted by nitroglycerin); – (2) it is severe and described as frank pain and of new onset (i.e., within 1 month); and – (3) it occurs with a crescendo pattern (i.e., more severe, prolonged, or frequent than previously). • Some patients with this pattern of ischemic discomfort, especially those with prolonged rest pain, develop evidence of myocardial necrosis on the basis of the release of cardiac markers and thus have a diagnosis of NSTEMI. BRAUNWALD CLINICAL CLASSIFICATION OF UNSTABLE ANGINA CLASS DEFINITION DEATH OR MYOCARDIAL INFARCTION TO 1 YEAR* Severity Class I New onset of severe angina or accelerated angina; no rest pain 7.3% Class II Angina at rest within past month but not within preceding 48 hr (angina at rest, subacute) 10.3% Class III Angina at rest within 48 hr (angina at rest, subacute) 10.8%† Clinical Circumstances A (secondary angina) Develops in the presence of extracardiac condition that intensifies myocardial ischemia 14.1% B (primary angina) Develops in the absence of extracardiac condition 8.5% C (postinfarction angina) Develops within 2 weeks after acute myocardial infarction 18.5%‡ Meccanismi eziopatogenetici Possono agire isolatamente o congiuntamente • Complicanza di placca: trombosi non occlusiva su placca significativa ma non critica (es. stenosi 60%) – Trombo piastrinico – E’ il meccanismo di gran lunga più frequente • Spasmo su stenosi lieve, o in assenza di stenosi (Prinzmetal) – Più raro, ma spesso presente anche dopo un trombo piastrinico (trombossano) Meccanismi eziopatogenetici Possono agire isolatamente o congiuntamente • Ostruzione meccanica progressiva (crescita di placca) – Meccanismo tipico della restenosi dopo angioplastica o intrastent • Infiammazione o infezione • Aumento della richiesta di flusso da causa extracardiaca – Tireotossicosi – Anemia Schematic representation of the causes of unstable angina. Each of the five bars represents one of the etiologic mechanisms, and the red portion of the bar represents the extent to which the mechanism is operative. A, Most common form of unstable angina in which atherosclerotic plaque causes moderate (60 percent diameter) obstruction and acute thrombus overlying plaque causes very severe (90 percent diameter) narrowing. B, Mild coronary obstruction, adjacent to which there is intense (90 percent diameter) vasoconstriction. PLAQUE RUPTURE, FISSURE, OR EROSION. • Rupture or erosion of an atherosclerotic plaque with superimposed nonocclusive thrombus is by far the most common cause of UA/NSTEMI. • The type of plaque that ruptures, the so-called vulnerable plaques, are usually lesions with less than 50 percent • Plaque rupture can be precipitated by multiple factors, including – high plaque lipid content, – local inflammation causing breakdown of the thin shoulder of the plaque, – coronary artery constriction at the site of the plaque, – local shear stress forces, – platelet activation, and the status of the coagulation system (i.e., a potentially prothrombotic state), • all of which culminate in the formation of platelet-rich thrombi at the site of the plaque rupture or erosion and the resultant acute coronary syndrome Platelets initiate thrombosis at the site of a ruptured plaque: the first step is platelet adhesion (1) via the glycoprotein Ib receptor in conjunction with von Willebrand factor. This is followed by platelet activation (2), which leads to a shape change in the platelet, degranulation of the alpha and dense granules, and expression of glycoprotein IIb/IIIa receptors on the platelet surface with activation of the receptor, such that it can bind fibrinogen. The final step is platelet aggregation (3), in which fibrinogen (or von Willebrand factor) binds to the activated glycoprotein IIb/IIIa receptors of two platelets. Aspirin (ASA) and clopidogrel act to decrease platelet activation, whereas the glycoprotein IIb/IIIa inhibitors inhibit the final step of platelet aggregation. Vasocostrizione • Prinzmetal – Vasocostrizione di coronaria epicardica, focale, anche in assenza di lesione aterosclerotica • Vasocostrizione microcircolatoria • Vasocostrizione in presenza di placca – Serotonina, Trombossano A2 Quadro clinico • Circa 80% dei pazienti (45% di sesso femminile) hanno già precedenti di cardiopatia ischemica • Sintomatologia importante: senso di peso ma spesso vero dolore • Insorgenza a riposo o da sforzi lievi • Spesso sintomi collaterali neurovegetativi Diagnosi • Sintomi e quadro clinico • Elettrocardiogramma – Sottoslivellamento ST – Onde T • Markers cardiospecifici (cTroponina I e T) – Livello di rischio – Livello di necrosi INDICATORS OF INCREASED RISK IN UNSTABLE ANGINA History Advanced age (>65 years) Diabetes mellitus Post-myocardial infarction angina Prior peripheral vascular disease Prior cerebrovascular disease Clinical Presentation Braunwald Class II or III (acute or subacute rest pain) Braunwald Class B (secondary unstable angina) Heart failure/hypotension Electrocardiogram New/ST segment deviation 0.05 mV New T wave inversion 0.3 mV Left bundle branch block Cardiac Markers Increased troponin T or I or CK-MB Increased C-reactive protein (CRP) Angiogram Thrombus Stratificazione del rischio nelle sindromi coronariche acute Trattamento • Stabilizzare (“passivating”) la placca – Aspirina, Clopidogrel, UFH o LMWH • Correggere l’ischemia – Nitrati, beta-bloccanti, calcioantagonisti – Rivascolarizzazione • Angioplastica • Bypass • Prevenzione a lungo termine della progressione della malattia Pooled data from CAPTURE, PRISM-PLUS, and PURSUIT trials of unstable angina, showing benefit of glycoprotein IIb/IIIa inhibition during medical therapy only (left panel), during, and immediately after percutaneous coronary intervention (PCI, right panel). Meccanismi favorevoli degli inibitori della GP IIb-IIIa • Greater resolution of thrombus and improved coronary flow compared with aspirin and heparin • Reduced size of an evolving NSTEMI • Greater benefit of treatment when administered earlier relative to the onset of pain • Warning: No thrombolytics! Infarto miocardico acuto Diagnosi di dimissioni ospedaliere nel triennio 1996 - 1998 1996 Numero N° ricoveri per infarto miocardico Degenza media IMA N° ricoveri per angina pectoris Degenza media AP 77.033 1997 Tassi per 10000 abitanti 134,21 11,62 75.966 Numero 80.920 1998 Tassi per 10000 abitanti 140,7 10,93 132,35 7,68 75.509 Numero 88.773 Tassi per 10000 abitanti 154,15 10,39 131,29 7,19 79.236 137,59 6,46 N°cateterismi cardiaci 61.378 106,94 72.188 125,52 82.140 142,63 N° By-pass 19.327 33,67 23.318 40,54 25.927 45,02 N° PTCA 14.415 25,11 20.250 35,21 28.104 48,8 IMA = infarto miocardico acuto; AP = angina pectoris Fonte ISTISAN Sopravvivenza post-IMA SOPRAVVIVENZA 100 ! Angina stabile ! 80 + Direct PTCA, statine ! 60 ! 40 ! Bypass, PTCA, TL, ACE-I Bypass 20 ! 0 0 2 4 6 FOLLOW-UP (ANNI) 8 10 12 Infarto miocardico acuto • Il 50% della mortalità avviene in fase preospedaliera • Mortalità ospedaliera – – – – Epoca pre-UCIC: 25% UCIC: 15% Trombolisi: 5-8% PTCA primaria: < 5% (studi randomizzati) Patologia • • • • Complicanza di placca Formazione di trombo di piastrine e poi Formazione di trombo di fibrina Il trombo è occlusivo Schematic representation of the progression of myocardial necrosis after coronary artery occlusion. Necrosis begins in a small zone of the myocardium beneath the endocardial surface in the center of the ischemic zone. This entire region of myocardium (dashed outline) depends on the occluded vessel for perfusion and is the area at risk. Note that a very narrow zone of myocardium immediately beneath the endocardium is spared from necrosis because it can be oxygenated by diffusion from the ventricle. Temporal sequence of early biochemical, ultrastructural, histochemical, and histological findings after onset of MI. At the top of the figure are schematically shown the time frames for early and late reperfusion of the myocardium supplied by an occluded coronary artery. For approximately one-half hour after the onset of even the most severe ischemia, myocardial injury is potentially reversible; after that there is progressive loss of viability that is complete by 6 to 12 hours. The benefits of reperfusion (both early and late) are greatest when it is achieved early, with progressively smaller benefits occurring as reperfusion is delayed. Fisiopatologia Fisiopatologia • Funzione sistolica • Funzione diastolica • Aritmie – Precoci – Tardive • Complicanze Disfunzione ventricolare • Nella zona infartuata • A distanza – Altre lesioni ischemizzanti – Circolo collaterale • Disfunzione sistolica – Ipocinesia – Acinesia – Discinesia • Disfunzione diastolica – Ischemia, Edema, Fibrosi Infarto miocardico acuto Funzione ventricolare 0,70 0,65 0,60 0,55 0,50 0,45 0,40 Frazione di Eiezione 0,35 0,30 0,25 0,20 -10 0 10 20 30 INFARCT SIZE (%) 40 50 60 100 90 80 P <<0,001 70 P = 0,5 60 VOLUME (ml/m2) LEFT VEN 50 40 30 0 10 20 30 40 INFARCT SIZE (% ) 50 60 72-hrs 6-months Infarto miocardico Funzione ventricolare in fase acuta e a 6 mesi 0,8 0,7 0,6 0,5 0,4 Frazione di Eiezione 0,3 0,2 0,1 -10 0 10 20 30 INFARCT SIZE (%) 40 50 60 Infarto acuto Infarto a 6 mesi Aritmie • Ipercinetiche precoci: da focus ectopico – Extrasistoli e tachicardia ventricolare – Fibrillazione ventricolare (morte improvvisa!) • Ipercinetiche tardive – Come sopra, ma da rientro • Ipocinetiche – Blocco atrioventricolare, anche completo, particolarmente nell’infarto inferiore (coronaria destra) Objective documentation of the circadian pattern in the onset of myocardial infarction (MI). Dolore nell’infarto • • • • Insorgenza brusca o graduale, non da sforzo Durata almeno 20’-30’ Insensibile alla NTG Accompagnato da fenomeni neurovegetativi (nausea, vomito, diarrea) • Esprime ischemia continuata: finché c’è dolore, c’è miocardio a rischio! • Scompare quasi subito col ripristino del flusso coronarico Presentazioni atipiche • “De novo” o peggioramento di insufficienza cardiaca congestizia • Semplice angina senza particolare gravità • Localizzazione anomala del dolore (solo dorsale, mandibola) • Sintomi neurologici centrali (ictus da brusca riduzione del flusso cerebrale) • Ansia e agitazione inspiegabile • Psicosi acuta • Sincope; Astenia invincibile • Indigestione acuta • Embolizzazione periferica Obiettività clinica • Il paziente è sofferente, ansioso, spesso sudato e pallido • Pressione arteriosa indifferente: spesso ipotensione, naturalmente sempre presente nei pazienti in shock • All’ascoltazione possibili 3. e 4. tono • Possibili sfregamenti pericardici a partire dalla terza giornata • Ascoltazione toracica: possibili rantoli in presenza di scompenso cardiaco Hyperacute phase of extensive anterior-lateral myocardial infarction. Marked ST elevation melding with prominent T waves is present across the precordium, as well as in leads I and aVl. ST depression, consistent with a reciprocal change, is seen in leads III and aVf. Q waves are present in leads V3 through V6. Marked ST elevations with tall T waves caused by severe ischemia are sometimes referred to as a monophasic current-of-injury pattern. A paradoxical increase in R wave amplitude (V2 and V3 ) may accompany this pattern. This tracing also shows left axis deviation with small or absent inferior R waves, which raises the possibility of a prior inferior infarct. Sequence of depolarization and repolarization changes with (A) acute anterior-lateral and (B) acute inferior wall Q wave infarctions. With anterior-lateral infarcts, ST elevation in leads I, aVl, and the precordial leads may be accompanied by reciprocal ST depression in leads II, III, and aVf. Conversely, acute inferior (or posterior) infarcts may be associated with reciprocal ST depression in leads V1 to V3. Variability of electrocardiogram (ECG) patterns with acute myocardial ischemia. The ECG may also be normal or nonspecifically abnormal. Furthermore, these categorizations are not mutually exclusive. For example, a non-Q-wave infarct can evolve into a Q wave infarct, ST elevation can be followed by a non-Q-wave infarct, or ST depression and T wave inversion can be followed by a Q wave infarct. Plot of the appearance of cardiac markers in blood versus time after onset of symptoms. Peak A, early release of myoglobin or CK-MB isoforms after AMI; peak B, cardiac troponin after AMI; peak C, CK-MB after AMI; peak D, cardiac troponin after unstable angina. Data are plotted on a relative scale, where 1.0 is set at the AMI cutoff concentration Markers di danno miocardico • Oggi si usano le troponine (cTnI e cTnT), estremamente specifiche (la concentrazione normale è zero) • Essenziale effettuare misurazioni seriate (6-12 ore) almeno fino al picco, poi più distanziate • Altri markers sono le CPK, meglio gli isoenzimi (BB, MM, MB) • Concomitanti alterazioni di AST e ALT, LDH • CPK e Tn sono usate per stimare le dimensioni dell’area necrotica Trattamento • Il trattamento inizia nella fase preospedaliera – Rapido riconoscimento dei sintomi, ECG a distanza • Limitare l’area infartuale – Riperfondere al più presto • Trombolisi preospedaliera – Farmaci antiischemici • Proteggere dalle aritmie potenzialmente letali – Ricovero in UCIC • Prevenire il rimodellamento • Prevenire la progressione della malattia Riperfusione miocardica • Va eseguita al più presto, preferibilmente entro tre ore dall’inizio dei sintomi; di scarsa utilità dopo 6 ore – Trombolisi controindicata dopo 12 ore • Oggi si tende ad ottenerla mediante angioplastica con stent (angioplastica primaria) • Trombolisi possibile se il tempo necessario per effettuare un’angioplastica è superiore a 60’-90’ – Dipende anche dal tempo già trascorso e dalla gravità della presentazione clinica (shock) Relationship between coronary blood flow and mortality in AMI Time-Dependent Benefit of Reperfusion Therapy 100 Reimer/Jennings 1977 Bergmann 1982 GISSI-I 1986 % Benefit 80 60 40 20 0 0 2 4 6 8 Reperfusion Time (hours) Adapted from Tiefenbrunn AJ, Sobel BE. Circulation. 1992;85:2311-2315. 10 12 Trattamento • • • • Nitroderivati in infusione Acido acetilsalicilico Eparina Betabloccanti – Evitare i calcioantagonisti • Possibilmente inserire al più presto – Ace-Inibitori – Statine • Trattamento del dolore (anche oppioidi) • Trattamento dell’insufficienza ventricolare sinistra Prevenzione del rimodellamento • Limitare l’estensione della necrosi – Rivascolarizzazione precoce! • Trattamento dell’ischemia residua • Pervietà dell’IRA (?) • Trattamento medico – – – – ACE-inibitori Bloccanti del recettore dell’angiotensina II Beta-bloccanti Nitrati Complicanze • Shock cardiogeno – Mortalità 80% • Rottura di cuore – Mortalità 100% • Insufficienza mitralica acuta • Rottura del setto interventricolare • Sindrome di Dressler (a distanza) – Non confondere con angina precoce postinfartuale Cause di morte per infarto miocardico acuto • Shock cardiogeno • Rottura di cuore • Aritmie – Oggi raro perché i pazienti sono ricoverati in UCIC • Dopo rivascolarizzazione efficace non si verifica rottura di cuore Dimissione • Oggi circa in decima giornata • Valutazione del rischio complessivo • Valutazione di: – Funzione ventricolare – Aritmie – Ischemia residua • Angina precoce postinfartuale TIMI Risk Score for STEMI for predicting 30-day mortality. STE = ST elevation; LBBB = left bundle branch block; h/o = history of; HTN = hypertension. Impact of left ventricular function on survival after myocardial infarction. The curvilinear relationship between left ventricular ejection fraction (LVEF) for patients treated in the thrombolytic era is shown.
Scaricare