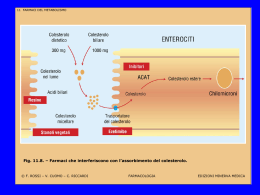
NUOVE STRATEGIE NEL TRATTAMENTO DELLA DISLIPIDEMIA Prof. Paolo de Caprariis MAL 1 Altered coagulation And fibrinolysis MAL 2 Lipoproteina 2 Sets of Lipoproteins 0.95 Density (g/mL) ApoA1 Apo B 1.006 1.02 1.06 1.10 1.20 5 MAL 10 20 40 Diameter (nm) 60 80 1000 4 Atherogenic Cholesterol Load Density (g/ml) 0.95 1.006 1.02 1.06 1.10 1.20 5 10 20 40 60 80 1000 Diameter (nm) MAL 5 Two Types of Lipoproteins are Atherogenic in Humans Apo B100 containing LDL Hepatic Apo B48 containing Chylomicron Remnants B100 B48 TG TG CE CE TG Intestinal Apolipoprotein B fragments Cholesteryl ester MAL 6 Atherosclerosis is linked to a Desequilibrium between Protective and Atherogenic Lipoproteins Apo B VLDL - IDL - LDL Atherogenic Transport Apo AI HDL MAL Anti Atherogenic Transport 7 FARMACI IMPIEGATI NELLE DISLIPIDEMIE Niacina, 1955 Resine sequestranti acidi biliari, 1961 Fibrati, 1967 Statine (inibitori HMG-CoA reduttasi), 1987 Inibitori dell’assorbimento del Colesterolo (ezetimibe), 2002 Terapia combinata, 2004-2005 NIACINA o VITAMINA B3 o PP Primo farmaco ipolipemizzante introdotto nel 1955. COMPLESSO DELLA VITAMINA B IDROFILO Sembra che agisca inibendo la lipolisi nel tessuto adiposo, con conseguente riduzione della sintesi epatica di VLDL Effetti collaterali: vasodilatazione, prurito al viso ed alle parti superiori del tronco, eritema, vampate Altri effetti collaterali importanti sono a carico del fegato, con aumento delle transaminasi e possibilità di ittero,dolore epigastrico,nausea,vomito, diarrea Niacin is Available in a Number of Different Formulations According to the Speed of Drug Release. Formulations that Differ in Time of Release May Have Different Lipid Effects and Vary in their Adverse Reaction Profiles Efficacy Hepatotoxicity Knopp R. Am J Cardiol 2000;86:51-56 Flushing DRUG DESCRIPTION NIASPAN (niacin tablet, film-coated extended-release), contains niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the following structural formula: NIASPAN is an unscored, medium-orange, film-coated tablet for oral administration and is available in three tablet strengths containing 500, 750, and 1000 mg niacin. NIASPAN tablets also contain the inactive ingredients hypromellose, povidone, stearic acid, and polyethylene glycol, and the following coloring agents: FD&C yellow #6/sunset yellow FCF Aluminum Lake, synthetic red and yellow iron oxides, and titanium dioxide. 11 Conjugated Pathway Non Conjugated Pathway Comparison of the Effect of Niaspan and Immediate Release Nicotinic Acid on Plasma Lipids and Lipoproteins 8 weeks N=223 Carlson L.A. J.Internal Medicine 2005;258:94-114 Mechanisms of Action of Nicotinic Acid Knopp R. NEJM 1999;341:498-511 Flushing Out the Role of GPR109A (HM74A) in the Clinical Efficacy of Nicotinic Acid Pike N. JCI 2005;115:3400-3403 Vit E 800UI Vit C 1000mg Beta Carotene 25mg Selenium 100ug Simva 10-40mg Niacor 2000mg N: 34 39 Brown G.NEJM 2001;345:1583-1592 33 40 Brown G.NEJM 2001;345:1583-1592 Wolfe M. Am.J Cardiology 2001;87:476-489 Simcor SIMCOR ® è indicato per ridurre elevate totale-C, C-LDL, Apo B, non-HDL-C, TG, o di aumentare il colesterolo HDL nei pazienti con ipercolesterolemia primaria e dislipidemia mista quando il trattamento con simvastatina in monoterapia o niacina monoterapia a rilascio prolungato è considerato inadeguato, e TG nei pazienti con ipertrigliceridemia quando il trattamento con simvastatina in monoterapia o niacina a rilascio prolungato in monoterapia è considerata inadeguata. http://www.drugs.com 19 Simcor Dosage and Administration Simcor should be taken as a single daily dose at bedtime, with a low fat snack. Patients not currently on niacin extended-release and patients currently on niacin products other than niacin extended-release should start Simcor at a single 500/20 mg tablet daily at bedtime. Patients already taking simvastatin 20-40 mg who need additional management of their lipid levels may be started on a Simcor dose of 500/40 mg once daily at bedtime. The dose of niacin extended-release should not be increased by more than 500 mg daily every 4 weeks. The recommended maintenance dose for Simcor is 1000/20 mg to 2000/40 mg (two 1000/20 mg tablets) once daily depending on patient tolerability and lipid levels. The efficacy and safety of doses of Simcor greater than 2000/40 mg daily have not been studied and are therefore not recommended. If Simcor therapy is discontinued for an extended period of time (> 7 days), re-titration as tolerated is recommended. Simcor tablets should be taken whole and should not be broken, crushed, or chewed before swallowing. MAL 20 RESINE LEGANTI I SALI BILIARI Sono disponibili due molecole: COLESTIRAMINA COLESTIPOLO CLORIDRATO Dal punto di vista chimico sono resine che legano anioni Meccanismo d’azione: Le resine legano gli acidi biliari scambiando ioni Cl- con cariche negative Effetti collaterali: stipsi, nausea Interazioni farmacologiche: diverse classi di vitamine anticoagulanti orali glicosidi cardioattivi diuretici β-bloccanti antibiotici Colesevelam Colesevelam is a bile acid sequestrant administered orally. It is developed by Genzyme and marketed in the US by Daiichi Sankyo under the brand name WelChol and elsewhere by Genzyme under the tradename Cholestagel. Clinical use Colesevelam is indicated as an adjunct to diet and exercise to reduce elevated low-density lipoprotein cholesterol (LDL-C) in patients with primary hyperlipidemia as monotherapy and to improve glycemic control in adults with type 2 diabetes mellitus, including in combination with a statin. Colesevelam is one of the bile-acid sequestrants, which along with niacin and the statins are the three main types of cholesterol-lowering agents. The statins are considered the first-line agents. This is because of side effects from the other two types, including bloating and constipation (bile-acid sequestrants) and skin flushing (niacin). These side effects often lead to low patient compliance. MAL 22 Constituents The compounds which constitute the polymer colesevelam are: N-prop-2-enyldecan-1-amine; trimethyl-[6-(prop-2-enylamino)hexyl]azanium; prop-2-en-1-amine; 2-(chloromethyl)oxirane; hydrogen chloride; chloride. 23 How it works Colesevelam is part of a class of drugs known as bile acid sequestrants. Colesevelam hydrochloride, the active pharmaceutical ingredient in Welchol, is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. As the bile acid pool becomes depleted, the hepatic enzyme, cholesterol 7-α-hydroxylase, is upregulated, which increases the conversion of cholesterol to bile acids. This causes an increased demand for cholesterol in the liver cells, resulting in the dual effect of increasing transcription and activity of the cholesterol biosynthetic enzyme, HMG-CoA reductase, and increasing the number of hepatic LDL receptors. These compensatory effects result in increased clearance of LDL-C from the blood, resulting in decreased serum LDL-C levels. Serum TG levels may increase or remain unchanged. It is not yet known how Colesevelam works to help control blood sugar in people with type 2 diabetes. However, it is clear that the drug works within the digestive tract, since it is not absorbed into the rest of the body. 24 Cholesterol Since Colesevelam can lower total and LDL cholesterol levels (along with raising HDL -- cholesterol), a person can decrease his or her risk of developing certain health problems in the future by taking it. In previous clinical research studies, people taking 3,800 mg to 4,500 mg of Colesevelam daily were able to: Reduce LDL cholesterol by 15 to 18 percent Reduce total cholesterol by 7 to 10 percent Raise HDL cholesterol by 3 percent. The combination of Colesevelam with a HMG-CoA reductase inhibitor (known more commonly as a statin) can further lower cholesterol levels. MAL 25 DERIVATI DELL’ACIDO FIBRICO: CLOFIBRATO E GEMFIBROZIL • Riduzione dei livelli di VLDL •Modesto aumento delle HDL • Effetto variabile sulle LDL Il clofibrato attiva la lipasi a livello endoteliale Farmacocinetica • Ben assorbiti per os. •Elevatissimo legame (95%) con l’albumina plasmatica. •Generalmente ben tollerati, con scarsi effetti collaterali gastrointestinali. ATTENZIONE alla competizione con altri farmaci (es. anticoagulanti orali) per i siti di legame alle proteine plasmatiche Fibrati Cl O O OiPr O FENOFIBRATO Cl O O OiPr O Steiner G. Atherosclerosis 2005;182:199-207 Rate of Rhabdomyolysis With Fenofibrate + Statin Versus Gemfibrozil + Statin Jones P. Am.J. Cardiol. 2005;95:120-122 Fenofibrate Resulted in a 33 Times Lower Rhabdomyolysis Reporting Rate than Did Gemfibrozil O Cl O O FENOFIBRATE O Pri Jones P. Am.J. Cardiol. 2005;95:120-122 Grundy S. Am.J. Cardiology 2005;95:462-468 Structural Mechanism for Statin Inhibition of HMG-CoA Reductase Simvastatin Type 1 Butyryl group MAL Atorvastatin Type 2 Fluorophenyl group 32 Le statine inibiscono la biosintesi del mevalonato HMG-CoA HMG-CoA reduttasi Mevalonato STATINE Geranyl-difosfato Geranylgeranyl difosfato Ubiquinone Farnesyl-difosfato Squalene Colesterolo Dolicolo Anti-hyperlipidemic Drugs - Statins HO R' O HO R R O O O O O CH2CH2 CH3 CH3 R COONa R OH CH2CH2 CH3 CH3 HO R'' R' Mevastatin H Lovastatin H Simvastatin CH3 R'' Pravastatin H CH3 CH3 34 Anti-hyperlipidemic Drugs - Statins _ COO Ca + HO HO OH COONa OH F OH F F N H HO COONa CH3 CH3 CH3 CH3 CH3 O H3C O NH CH3 N H3C Atorvastatin N CH3 Cerivastatin Fluvastatin _ + COO Ca HO _ + COO Ca HO OH OH F CH3 F CH3 O H3C S N N N N CH3 O Rosuvastatin Pitavastatin 35 Anti-hyperlipidemic Drugs - Statins Rationale – competitive binding O HO O HO COONa OH HO COOH SCoA O For example, Mevastatin Lovastatin Simvastatin For example, Fluvastatin Atorvastatin Cerivastatin HMG CoA substrate 36 Anti-hyperlipidemic Drugs - Statins Pharmacokinetic properties of statins – case of cerivastatin Bioavail. Dosage (mg) Protein Binding Metabolites Atorvastatin ~14% 10 – 80 >98% Active Cerivastatin ~60% 0.2 – 0.3 >99% Active Fluvastatin ~24% 10 – 80 98% Active Lovastatin ~5% 10 – 80 >95% Pravastatin ~17% 10 – 40 ~50% Simvastatin ~5% 10 - 80 ~95% Typically all statins possess side effects. The most dominant side effect, cited in the withdrawal of cerivastatin, is rhabdomyolysis (lysis of rhabdomyose) or weakening of skeletal muscles. 37 MECCANISMO D’AZIONE DELLE STATINE STATINE Riduzione attività HMG CoA reduttasi Deplezione del pool di colesterolo nell’epatocita Aumento espressione recettori LDL epatici Aumento clearance LDL circolanti Diminuita produzione di VLDL Alterata composizione delle VLDL STATINE DI I GENERAZIONE MEVASTATINA e’ stata la prima sostanza scoperta, è stato isolata da colture di specie di Penicillum LOVASTATINA è un analogo della mevastatina, con aggiunto un gruppo metile E’ stato isolato da colture di Aspergillus. Molecola lipofila, emivita: 2-3 ore. PRAVASTATINA è anch’esso un analogo della mevastatina, con aggiunto un gruppo idrossilico. Molecola idrofila, emivita: 1 ora. STATINE DI II GENERAZIONE SIMVASTATINA di derivazione semisintetica. Molto simile alla lovastatina. Indicata: ipercolesterolemia primaria Ipercolesterolemia familiare nella variante eterozigote iperlipidemia mista (tipo IIa e IIb) STATINE DI III GENERAZIONE FLUVASTATINA è una molecola sintetizzata chimicamente. Molecola idrofila, ha una breve emivita. STATINE DI IV GENERAZIONE ATORVASTATINA di derivazione sintetica. Molecola lipofila, con una lunga emivita (13-16 ore). Indicata: - ipercolesterolemia familiare nella variante omozigote - ipercolesterolemia primaria - iperlipidemia mista (tipo IIa IIb) CERIVASTATINA di derivazione sintetica. Molecola idrofila, emivita: 2-3 ore. E’ circa 100 volte più potente rispetto alle altre statine. Ritirata dal commercio perché si sono manifestati casi di rabdomiolisi mortale per sovradosaggio o per l’associazione con altri farmaci ipocolesterolemizzanti. ROSUVASTATINA (3R, 5S) O HO •Gruppo polare metan-sulfonico •La più potente statina: 10-80 mg/dl riduzione LDL-C da 34%-65% e fino a 90% /2 sett. •Diminuzione apolipoproteina b e trigliceridi 10-35% •Aumento HDL da 9-14% •Basso rischio di interazioni con altri farmaci Ca O OH F CH 3 CH 3 N N H 3C S O N O CH 3 Prostata Testicoli Surrene Tiroide Cervello Cervelletto Occhio Milza Ileo Cuore Polmone Rene Fegato trasporto attivo per alta affinità con un sistema organo-specifico (OATPs :Organic Anion Transport Proteins) 0,0 0,2 0,4 0,6 CLUptake (mL/min/g tessuto) 0,8 1,0 SINTESI SIMVASTATINA SINTESI SIMVASTATINA SINTESI SIMVASTATINA FARMACOCINETICA Sono somministrate per os ed hanno un assorbimento variabile. Simvastatina:85% di assorbimento Pravastatina:30% assorbimento Fluvastatina:assorbita quasi completamente Hanno tutte un esteso effetto di primo passaggio che, per la lovastatina e la simvastatina, serve per dare origine al farmaco attivo. Sono strettamente legate alle proteine plasmatiche (50% pravastatina, 95% le altre) Escrete quasi completamente per via intestinale. Vengono generalmente somministrate in unica dose serale, perché la sintesi di colesterolo segue un ritmo circadiano, aumentando la notte. Differenze farmacocinetiche delle statine: metabolismo epatico Pravastatina Rosuvastatin a Fluvastatina Lovastatina Simvastatina Cerivastatina Atorvastatina 50 – 80% <5% CYP2C9 CYP3A4 CYP2C8 Prodotti di degradazione attivi o inattivi FDA Approves LIVALO(R) For Primary Hypercholesterolemia And Combined Dyslipidemia Pitavastatin LIVALO(R) (pitavastatin), a potent HMG-CoA reductase inhibitor (statin). MAL 48 Livalo is a fully synthetic and highly potent statin engineered in Japan. Livalo differs from other, currently available statins in the U.S. in that it has a unique cyclopropyl group on the base structure. This cyclopropyl group contributes to a more effective inhibition of the HMG-CoA reductase enzyme to inhibit cholesterol production, and potentially affords greater low-density lipoprotein cholesterol (LDL-C) clearance and reduction of plasma cholesterol. Importantly, pitavastatin is only minimally metabolized by the liver through the cytochrome P450 pathway, through which many other medications are metabolized. In pivotal Phase III trials, Livalo effectively reduced LDL-C and improved other parameters of lipid metabolism in special patient populations, including the elderly, patients with diabetes and patients at higher cardiovascular risk. The overall safety and tolerability of Livalo are consistent with other commonly prescribed statins. Livalo is expected to launch in the U.S. during Q1 of 2010 and will be available in 3 low dosages (1 mg, 2 mg and 4 mg). After a thorough review of the statin market, KPA is also seeking a co-promotion partner in order to broaden the reach of KPA's rapidly growing internal sales force. Partnering with another organization to expand the sales efforts for this product is aligned with KPA's long-term vision to become a leader in the cardiometabolic therapeutic arena. Since its launch in Japan, South Korea, Thailand and China, Livalo has been successfully used in these countries to treat primary hypercholesterolemia and combined dyslipidemia, and has accumulated millions of patient-years of exposure. It is frequently prescribed in these countries as first-line therapy for a broad range of patients including the elderly, patients with diabetes and those whose treatment is complicated by concurrent disease and concomitant medications. Selected Drugs That May Increase Risk of Myopathy When Used Concomitantly with Statins MAL Adapted from Corsini A. Pharmacol.Ther. 1999;84:413-428 51 INTERAZIONI FARMACOLOGICHE CON LE STATINE: le principali classi di farmaci a rischio INTERAZIONI FARMACOCINETICHE: Inibitori CYP 3A4: Ciclosporina, Eritromicina, Ritonavir, Fluconazolo, fluoxetina, pompelmo (> la concentrazione plasmatica delle statine) Induttori CYP 3A4: Barbiturici, Carbamazepina, Fenitoina, Rifampicina (< la concentrazione plasmatica di statine) Inibitori CYP 2C9: Amiodarone, Cimetidina, Isoniazide, Chetonazolo (> concentrazione plasmatica di fluvastatina) Induttori CYP2C9: Barbiturici,Carbamazepina, Fenitoina, Rifampicina (< concentrazioni plasmatiche fluvastatina) Antibiotici macrolidi: Eritromicina, Claritromicina Antifungini azolici: ketoconazolo Calcio-antagonisti Acido nicotinico Benzodiazepine: Diazepam, Midazolam Anticoagulanti cumarinici : warfarin INTERAZIONI FARMACODINAMICHE: Gemfibrozil – fibrati Altre formulazioni combinate Co-somministrazione: statina + niacina Advicor ( Nicostatin) combinazione di lovastatina e niacina <LDL del 47% >HDL del 41% Aumento glicemia e acido urico con conseguenti anomalie Co –somministrazione: statina + resine Effetti collaterali gastrointestinali: costipazione, gonfiore, flautolenza, dolori addominali Co-somministrazione : statina + fibrati Gemfibrozil > concentrazione statine nel sangue alzando il potenziale di tossicità si sostituì con il fenofibrato. Controllo periodico: Alanina aminotransferasi (ALT) Aspartato aminotransferasi (AST) Creatina kinasi (CK) Usare cautamente un trattamento combinato in pazienti con età superiore a 70 anni Se appaiono sintomi muscolari sospendere la terapia Non somministrare in pazienti con insufficienza epatica Whole Body Cholesterol Homeostasis is Maintained Through 3 Major Pathways Intestinal Absorption LDL-C de novo Synthesis MAL Bays H. Expert Opin. Investig. Drugs 2002; 11: 1587-1604 Biliary Excretion 54 Intestinal Cholesterol Absorption is a Multistep Process that is Regulated by Multiple Genes Lumen Enterocyte Sterol Influx Transporter Lammert F. Gastroenterology 2005;129:718-734 Lymph Complementary Actions of Statins and Selective Cholesterol Absorption Inhibitors Assorbimento di Colesterolo nell’Intestino 300–700 mg 1000 mg Plant stanols NPC1L1 Ezetimibe: Parametri Farmacocinetici Assorbimento Rapido,dopo somministrazione orale Picco di concentrazione plasmatica di metabolita attivo in 1–2 ore Metabolismo Rapidamente metabolizzato a metabolita attivo: ezetimibeglucuronide Eliminazione attraverso le feci Emivita ~22 ore / dose giornaliera Ezetimibe OH OH N F O Glucuronidazione F OGluc OH N F Glucuronide O F Synthesis Ezetimibe Effetti dell’ Ezetimibe sull’ Assorbimento del Colesterolo % Assorbimento Colesterolo 2 settimane 80 54% Riduzione dell’assorbimento di colesterolo con l’ezetimibe 70 60 50 49.8% 40 30 22.7% 20 10 Individuali livelli di assorbimento Principali livelli di assorbimento 0 Placebo Ezetimibe Effetti dell’ Ezetimibe sull’ Aterogenesi Sezione trasversale dell’arteria coronaria Control Nuovi approcci: Ezetimibe La associazione di Ezetimibe con statina agisce attraverso la duplice inibizione della sintesi di colesterolo a livello epatico e di assorbimento di colesterolo a livello intestinale. La associazione di Ezetimibe 10 mg con qualsiasi statina al dosaggio di 10 o 20 mg produce una riduzione di LDLcolesterolo sovrapponibile a quella ottenibile con il dosaggio massimo della statina. Oltre il 70% dei pazienti che non hanno raggiunto l’obbiettivo terapeutico in monoterapia con statine, lo raggiungono se si associa Ezetimibe Efficacy of LDL-C Lowering with Ezetimibe/Simvastatin Compared with Simvastatin Alone In Patients with Primary Hypercholesterolemia INEGY Inegy è indicato come terapia aggiuntiva alla dieta in pazienti con ipercolesterolemia primaria (eterozigote familiare e non-familiare) o con iperlipidemia mista ove sia indicato l´uso di un prodotto di associazione: Pazienti non controllati adeguatamente con una statina da sola. Pazienti già trattati con una statina ed ezetimibe. Inegy contiene ezetimibe e simvastatina. E´ stato dimostrato che la simvastatina (20-40 mg) riduce la frequenza degli eventi cardiovascolari (vedere paragrafo 5.1). Non sono stati completati gli studi per dimostrare l´efficacia di Inegy o di ezetimibe nella prevenzione delle complicazioni dell´aterosclerosi. Ipercolesterolemia familiare omozigote (IF omozigote) Inegy è indicato come terapia aggiuntiva alla dieta in pazienti con ipercolesterolemia familiare omozigote. I pazienti possono essere sottoposti anche ad ulteriori misure terapeutiche (per MALesempio, l´aferesi delle lipoproteine a bassa densità [LDL]). 64 INEGY Gruppo farmacoterapeutico: altri ipocolesterolemizzanti ed ipotrigliceridemizzanti. Codice ATC: C10A X Inegy (ezetimibe/simvastatina) è un prodotto ipolipemi zzante che inibisce selettivamente l´assorbimento intestinale del colesterolo e dei relativi steroli vegetali e inibisce la sintesi endogena del colesterolo. Meccanismo d´azione:InegyIl colesterolo plasmatico è derivato dall´assor bimento intestinale e dalla sintesi endogena. Inegy contiene ezetimibe e simvastatina, due composti ipolipe mizzanti con meccanismi d´azione complementari. MAL 65 Omega-3 Fatty Acids: The Basics What are Omega-3 fatty acids? What are common dietary sources? AHA Recommendations • For patients with documented CHD, about 1g of EPA+DHA per day – Capsules Low Potency - 300 mg EPA+DHA/g (Typical drug store capsules) High Potency - 500-700 mg EPA+DHA/g (CardioTabs, Triomega, OmegaRx) Pharmaceutical – 850 mg EPA+DHA/g (Omacor®, Reliant Pharmaceuticals) – Cod Liver Oil • 1 tsp (RDA for Vit. D; 2x RDA Vit. A) OMACOR® (omega-3-acid ethyl esters) reduced triglycerides by a median of 45% while raising HDL-C by 9% OMACOR reduced non–HDL-C by 14% overall,) The placebo group had no significant changes from baseline for any of the above lipid parameters Every attempt should be made to control serum TG levels with appropriate diet, exercise, weight loss in overweight patients, and control of any medical problems (such as diabetes mellitus and hypothyroidism) that may be contributing to the patient’s TG abnormalities. Combination Lipid Therapy Options Vasudevan A. Curr. Athero. Rep. 2006;8:76-84 While cholesterol is necessary for many biological functions, too much of it increases our risk of cardiovascular diseases. Most of us have an unfortunate appetite for foods rich in cholesterol and some of us are genetically predisposed to handle it worse than others, so the scientific community is working hard to help us control cholesterol levels. As cholesterol is insoluble in blood, it must be carried around in the form of lipoproteins. One particular type of transporter, HDL (high-density lipoprotein), has been the focus of many research groups. HDL carries cholesterol away from arteries to the liver, where the cholesterol can be excreted or reused. Having more HDL is beneficial to the cardiovascular system, but raising HDL levels is difficult. HDL with surface proteins and cholesterol. Current drugs that induce our bodies to boost HDL concentration have negative side effects, leading some scientists to focus on mimicking HDL with synthetic nanoparticles instead. However, creating nanoparticles with the dynamic activities of HDL is a serious challenge. Scientists at Northwestern University report in an advanced issue of the Journal of the American Chemical Society that they have solved some of the problems involved in creating a synthetic HDL. Their idea was to use gold nanoparticles as the inner cores. The gold nanoparticles act as a scaffold that can be given the appropriate dimensions to resemble HDL. Apolipoproteins and phospholipids, which are present in natural HDL, can then be layered onto the nanoparticles to create a surface similar to HDL. With this in mind, the researchers created synthetic HDL nanoparticles with a diameter of about 18 nm, making them similar in size to the biological ones Each nanoparticle contained between 2 and 5 apolipoproteins and 80 to 160 phospholipids; chemical composition analysis showed that this surface constitution is analogous to natural HDL. The nanoparticles are also soluble in water, suggesting they can dissolve in blood and move to and from cells. To determine how well the synthetic HDL can bind cholesterol, the scientists used a fluorescent analogue of cholesterol for their studies. When the cholesterol analogues are in water, they are weakly fluorescent; when they are bound to the synthetic HDL, their fluorescence is increased. Using the fluorescence signal as an indicator, the authors determined that in a 5 nM solution of synthetic HDL, the binding affinity to cholesterol has a dissociation constant of 3.8 nM. This is the first time that a research group has published a value in this range for a synthetic HDL—no value is known for natural HDL, so it's impossible to make comparisons. The authors' approach—creating a nanoparticle that mimics the size and functions of biological HDL—is a good starting point for further investigations. The next steps in this line of research include determining how well this synthetic HDL transports cholesterol to the liver, testing the toxicity of the nanoparticles, and finding methods of introducing them inside the body. MAL 72 Anacetrapib Anacetrapib (codenamed MK-0859, Merck) is a CETP inhibitor being developed to treat hypercholesterolemia (elevated cholesterol levels) and prevent cardiovascular disease. It has been in Phase I clinical trials; preliminary results appear encouraging, although long-term safety data are lacking. At the 16th International Symposium on Drugs Affecting Lipid Metabolism (New York, Oct 4-7, 2007), Merck reported on a Phase IIb study. The eight week study reported dosage correlated reduction in LDL-C and increases in HDL-C levels with no corresponding increases in blood pressure in any cohort. The increase in HDL was particularly significant, averaging 44 percent, 86 percent, 139 percent and 133 percent at doses of 10 mg, 40 mg, 150 mg and 300 mg. 73
Scaricare