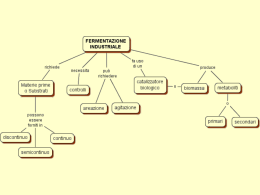
Un processo all’interfaccia • Per: O + ne- = R • 5 eventi separati devono verificarsi: – O devono essere trasportati con successo dal bulk della soluzione (transporto di massa) – O deve adsorbirsi in modo transiente sulla superficie dell’elettrodo (non-faradico) – dveve avvenire un trasferimento di carica tra elettrodo ed O (faradico) – R deve desorbirsi dalla superfiie elettrodica (nonfaradico) – R deve essere trasportato lontano dall’elettrodo nel bulk della soluzione (transporto di massa) Classificazione degli Elettrodi • si basa sulla natura e sul numero delle fasi tra cui avviene il trasferimento elettronico • 3 Classi: – Elettrodi di I specie – Elettrodi di II specie – Elettrodi di III specie Elettrodi di I specie • Metallo in contatto con suoi cationi o nonmetallo in contatto con suoi anioni • ESEMPI: – – – – – Cu2+ /Cu(s) Zn2+/Zn(s) Electtrodi nella pila Daniell SHE Ag+/Ag (Elettrodo di riferimento non acquoso) Cl-/Cl2(g)/Pt Elettrodi di I specie • Risposta dell’elettrodo data dalla equazione di Nernst (Nernstiano): RT EE ln aM z nF 0 • N.B.: elettrodi di Fe, Al, e W NON sono elettrodi di I specie – spessa ricopertura superficiale da parte degli ossidi Electrode of the Second Kind • Metal in contact with sparingly soluble salt of the metal • Common name: anion electrodes • EXAMPLES: – Ag/AgCl(s) – Hg/Hg2Cl2(s)/Cl- (saturated calomel electrode; SCE) Ag/AgCl, KCl Il sale insolubile è AgCl sottoposto all’equilibrio di solubilità: AgCl Ag Cl K PS [ Ag ][Cl ] a Ag aCl a Ag K PS aCl La reazione redox corrispondente è: Ag Cl e Ag Cl Essendo insolubile è presente come corpo di fondo o deposito sul metallo aAg+=1 Il potenziale redox è relativo alla coppia Ag/Ag+: E Ag / Ag E 0 Ag / Ag 0 E Ag / Ag E Ag / Ag E 0' Ag / Ag E 0 Ag / Ag 0' E Ag / Ag E Ag / Ag RT ln a Ag F RT RT RT K PS 0 ln aCl ln K PS E Ag / Ag ln F F aCl F RT ln K PS F RT ln aCl F Electrode of the Second Kind • Electrode response given by: E Ag / Ag E 0' Ag / Ag RT ln aCl F • NOTES: – anion activity determines potential – make great reference electrodes because of low solubility of salt (potential very stable) The Calomel Reference Electrode Electrode Acronym Hg(l)/Hg2Cl2(s)/KCl (0.1 M) Hg/Hg2Cl2(s)/KCl (1 M) Hg(l)/Hg2Cl2(s)/KCl (sat'd) Hg(l)/Hg2Cl2(s)/ NaCl (sat'd) NCE SCE SSCE Potential vs. SHE 0.3337 0.2801 0.2412 0.2360 Note: concentrations typically high concentrations small electrode doesn’t become polarized potential constant Electrode of the Third Kind • Electrodes that merely serve as sources or sinks for electrons • Common names: redox, inert, unattackable • EXAMPLES: – metals: Pt, Au, GC, graphite, HOPG, Hg – semiconductors: Si, GaAs, In-SnO2/glass • Response: – for Pt in contact with Fe2+, Fe3+ in solution: – E = E0- 0.059 (V) log ([Fe2+]/[Fe3+]) Electrode of the Fourth Kind • Electrodes that cannot be classified as 1-3 • EXAMPLES: – Chemically modified electrodes (CME’s) Reference Electrodes • Purpose: provide stable potential against which other potentials can be reliably measured • Criteria: – stable (time, temperature) – reproducible (you, me) – potential shouldn’t be altered by passage of small current = not polarizable – easily constructed – convenient for use SHE Advantages Disadvantages • International standard • Convenience E0 0 V – Pt black easily poisoned by organics, • One of most sulfide, cyanide, etc. reproducible potentials – Hydrogen explosive + 1 mV – Sulfuric and hydrochloric strong acids Practical Reference Electrodes Aqueous • SCE • Ag/AgCl Nonaqueous • Ag+/Ag • pseudoreferences – Pt, Ag wires • Ferrocene SCE • Cl-(aq)/Hg2Cl2/Hg(l) • Hg22+ + 2e- = 2Hg(l) • E0 = 0.24 V vs. SHE @ 250C Advantages • Most polarographic data ref’d to SCE Disadvantages • Hg toxic • solubility of KCl temperature dependent dE/dT = 0.67 mV/K (must quote temperature) From BAS wwwsite: http://www.bioana lytical.com/ Ag/AgCl • Ag wire coated with AgCl(s), immersed in NaCl or KCl solution • Ag+ + e- = Ag(s) • E0 = 0.22 V vs. SHE @ 250C Advantages • chemical processing industry has standardized on this electrode • convenient • rugged/durable Disadvantages • solubility of KCl/NaCl temperature dependent dE/dT = -0.73 mV/K (must quote temperature) From BAS wwwsite: http://www.bioana lytical.com/ Ag+/Ag • Ag+ + e-= Ag(s) • requires use of internal potential standard Advantages • Most widely used • Easily prepared • Works well in all aprotic solvents: – THF, CAN, DMSO, DMF Disadvantages • Potential depends on – solvent – electrolyte (LiCl, TBAClO4, TBAPF6, TBABF4 • Care must be taken to minimize junction potentials From BAS wwwsite: http://www.bioana lytical.com/ Pseudo-References • Pt or Ag wire (inert) • Idea: in medium of high resistance, low conductivity, wire will assume reasonably steady, highly reproducible potential (+ 20 mV) • Advantage: no solution contamination • Limitation: must use internal potential standard (ferrocene) Can Aqueous References Be Used in Nonaqueous Media? • Yes with caution! – May be significant junction potentials • Requires use of internal standard – May be greater noise • Electrolyte may precipitate/clog electrode frit – Don’t forget about your chemistry • Chemistry may be water sensitive Electrodes • Metal – solid • Pt, Au, Ag, C – liquid • dropping mercury electrode (DME) • Semiconductors – Si, GaAs – In-SnO2/glass (optically transparent) Carbon • Paste – With nujol (mineral oil) • Glassy carbon (GC) – Amorphous • Pyrolytic graphite - more ordered than GC – Basal Plane – Edge Plane (more conductive) Electrode Materials • Different Potential Windows • Can affect electron transfer kinetics Electrodes • Size – Analytical macro • 1.6 - 3 mm diameter – Micro • 10-100 m diameter From BAS www-site: http://www.bioanalytical.com/ Electrode Geometry Geometry is critical and affects how the data are analyzed and interpreted Note: Geometric area < • Disk – area: r2 • wire (cylinder) – area: l(2 r) r2 • Mesh – optically transparent • Sheet effective surface area Cleanliness IS Next to Godliness in Electrochemistry • Working electrode must be carefully cleaned before each experiment – Mechanical • Abrasion with alumina or “diamond” polish – Chemical • Sonicate in Alconox • Soak in HNO3 – Electrochemical • Cycle in 0.5 M H2SO4 (Pt) Electrochemical Cleaning Taken from Table 4-7 in Sawyer, D.T.; Roberts, Jr., J.L. Experimental Electrochemistry for Chemists Wiley: New York, 1976.
Scarica