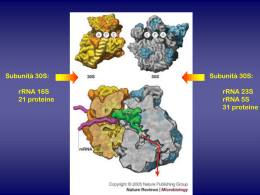
The Quinolones: Past, Present, and Future Vincent T. Andriole Yale University School of Medicine, New Haven, Connecticut Unlike some of the first antibiotics discovered during the past century, the quinolone class of antimicrobial agents was not isolated from living organisms but, rather, was synthesized by chemists. Chemical structure of the quinolone/naphthyridone ring system, showing sites that are modified in various agents, and the structures of the fluoroquinolones investigated. Atomo di fluoro in posizione 6 Pestova E et al. J. Antimicrob. Chemother. 2000;45:583-590 © 2000 The British Society for Antimicrobial Chemotherapy FARMACOCINETICA CHINOLONI In early studies, the quinolones were observed to have excellent oral absorption, good distribution in tissue, with excellent interstitial fluid levels, entry into phagocytic cells, and urinary concentrations that exceeded the MICs for many common pathogens. Key structural modifications resulted in improved pharmacokinetics (e.g., a longer elimination half-life, which permitted once-daily dosing and better tissue penetration) of some of the newest quinolones, including gatifloxacin, gemifloxacin, grepafloxacin, moxifloxacin, sitafloxacin, sparfloxacin, and trovafloxacin. Specific modifications included alkylation of the quinolones, which improved elimination half-life and penetration into tissue; the addition of 2 methyl groups to the C-7 piperazine ring, which increased oral efficacy; the addition of an amino group at C-5, which increased lipophilicity; and the addition of a halogen at position C-8, which improved in vivo activity. Clinical Infectious Diseases 2005; 41:S113–9 I chinoloni sono divisi in quattro generazioni in base al loro spettro di azione. Quelli delle prime generazioni sono quelli con uno spettro di azione più ristretto. 1a generazione 2a generazione 3a generazione 4a generazione Cinoxacina Acido nalidissico Acido ossolinico Acido piromidico Acido piemidico Rosoxacina Ciprofloxacina Enoxacina Fleroxacina Lomefloxacina Nadifloxacina Norfloxacina Oxafloxacina Gatifloxacina Grepafloxacina Sparfloxacina Clinafloxacina Garenoxacina Gemifloxacina Sitafloxacina Trovafloxacina Spettro di azione dei chinolonici 1° Generazione Acido nalidissico: Escherichia coli, Proteus, Shigella, Enterobacter, e Klebsiella. 2° Generazione Ciprofloxacina: Come quelli della prima generazione ed nei confronti della salmonella typhy 3° Generazione Gatifloxacina: Chlamydophila pneumoniae e Mycoplasma pneumoniae 4° Generazione Gemifloxacina: Moraxella catarrhalis, Acinetobacter lwoffii, Klebsiella oxytoca, Legionella pneumophila, Proteus vulgaris. - Chlamydia pneumoniae, Mycoplasma pneumoniae 2a Generazione 2a Generazione 3a Generazione 4a Generazione Mechanismo di Azione I chinoloni sono farmaci battericidi. Inibiscono la DNA girasi batterica detta anche topoisomerasi IV inibendo così la replicazione e la trascrizione del DNA batterico. I chinoloni entrano nel batterio attraverso dei canali detti porine e perciò vengono usati nel trattare infezioni batteriche di patogeni che si replicano all’interno di cellule umane quali la legionella ed il micoplasma pneumonie. Per molti batteri gram negativi la DNA girasi è il target di questi antibatterici mentre per molti gram positivi il target è la DNA topoisomerasi IV Le cellule eucariotiche non contengono ne DNA girasi e ne DNA topoisomerasi IV Effetto della DNA girasi Effetti collaterali dei fluorochinoloni Gli effetti collaterali dei fluorochinoloni possono essere di natura lieve e di breve durata o di natura severa e di lunga durata dopo che la terapia è stata terminata. Se gli effetti collaterali avvengono al livello del Sistema nervoso centrale (SNC) o del sistema nervoso periferico (SNP) o a livello del sistema muscolare la terapia deve essere interrotta immediatamente. Gli effetti collaterali dei fluorochinoloni sono i seguenti: Ansietà, dolore articolare, dolore muscolare, tendiniti, visione offuscata, perdita della memoria, diarrea, attacchi di panico, insonnia, rottura del tendine d’achille, tachicardia. Adverse events commonly associated with the fluoroquinolones include gastrointestinal and CNS toxicity (most frequently headache and dizziness), as well as other adverse events including ECG abnormalities (for example QT interval prolongation), disrupted glucose metabolism, phototoxicity, tendon and joint disorders, hypersensitivity and skin disorders, and hepatic toxicity. Expert Opin Drug Saf. 2012 Jan;11(1):53-69. Epub 2011 Sep 30. Cardiovascular and metabolic safety profiles of the fluoroquinolones. Pugi A, Longo L, Bartoloni A, Rossolini GM, Mugelli A, Vannacci A, Lapi F. University of Florence, Department of Pharmacology, Viale Pieraccini 6, 50139, Florence, Italy. [email protected] INTRODUCTION: Certain fluoroquinolones share similar indications of use. A comparison among Cardiovascular and metabolic (i.e., dysglycemia) safety profiles of the fluoroquinolones might be particularly useful for the prescribers' decision-making process as well as to hypothesize future researcher purposes. EXPERT OPINION: Cardiac alterations and blood glucose impairments might be associated with any fluoroquinolone. However, the benefit/risk profile of these agents could be stratified for the single compounds. Several predisposing factors, such as diabetes, heart illnesses and their related pharmacotherapies, might exacerbate the risk of potentially serious adverse events. In this context, the opportunity of the more appropriate choice among different fluoroquinolones could be applicable. We did find, however, an interaction between the combined use of fluoroquinolones and renin–angiotensin-system blockers CMAJ, July 9, 2013, 185(10) J Antimicrob Chemother. 1986 Nov;18 Suppl D:187-93. Ciprofloxacin: an overview of adverse experiences. Ball P. Abstract This review summarizes adverse reactions probably or possibly attributable to oral ciprofloxacin therapy in worldwide clinical experience involving over 6500 patients. In Europe and Japan the overall incidence of adverse reactions amongst patients receiving ciprofloxacin is reported to be 3.0% and 6.5%, respectively. An increased incidence (13.4%) has been reported from the U.S.A., possibly relating to the use of higher dosages. Very few reactions have necessitated withdrawal of treatment. The most common adverse effects involve the gastrointestinal system (2-8% of patients treated) and usually comprise nausea, vomiting, diarrhoea and abdominal discomfort. CNS effects are seen in 1-4% of patients but are usually minor dizziness or mild headache only. Hypersensitivity reactions, most commonly skin rashes or pruritus, affect about 1% of patients. There is little evidence of significant haematological or biochemical toxicity, other than a few reports of transient neutropenia and the finding, in a minority of clinical studies, of equally transient, usually trivial and invariably reversible elevations of serum aminotransferases. Serious, ciprofloxacin-related toxicity has been observed in only three patients: one who developed pseudomembranous colitis, another who developed interstitial nephritis and a third who had a grand-mal convulsion during concomitant administration of theophylline. Ciprofloxacin appears to have an excellent safety profile. Macrolidi I macrolidi sono un gruppo di farmaci la cui attività risiede nella sua struttura macrolide. The most important macrolide antibiotics are 14-, 15-, and 16-membered compounds. I classici farmaci macrolidi sono: 1) Eritromicina 2) Claritromicina 3) Azitromicina 4) Roxitromicina 5) Telitromicina 6) Spiramicina Eritromicina Claritromicina The molecular structure of erythromycin, the 14- membered prototype macrolide Azitromicina Azithromycin and to a lesser extent clarithromycin are noted for their high and prolonged concentrations at sites of infection, reaching tissue levels of 10–100-fold and 2–20- fold greater than serum concentrations, respectively. Both agents are also concentrated intracellularly by alveolar macrophages, attaining levels of approximately 400-fold (clarithromycin) and 800-fold (azithromycin) above serum concentrations. Drug delivery problems resulting from acid instability prompted the design of newer macrolides. These compounds include (i)clarithromycin, roxithromycin, dirithromycin, and the ketolides and fluoroketolides, all of which have a 14membered ring structure; (ii) the 15-membered azithromycin; and (iii) the 16-membered agents spiramycin, rokitamycin, and josamycin. Spettro d’Azione Macrolide antibiotics are generally used to treat respiratory and soft tissue infections caused by Gram-positive bacteria. They are also active against rickettsiae, chlamydiae, and Mycoplasma pneumoniae, as well as some Gram-negative bacterial pathogens, including Bacteroides fragilis, Bordetella pertussis, Campylobacter species, Haemophilus influenzae, Helicobacter pylori, Legionella pneumophila, Moxarella catarrhalis, and Neisseria species. I Ribosomi Le subunità ribosomiali dei batteri e degli eucarioti sono simili. I batteri hanno un’unità ribosomiale 70S che consiste di una subunità 30S (composta da RNA di 1540 nucleotidi e 21 proteine) e una 50S (che consiste di due subunità di RNA di 2900 + 120 nucleotidi e 34 proteine). Meccanismo di azione I macrolidi inibiscono la crescita batterica interferendo nella loro sintesi proteica. I macrolidi si legano alla subunità 50s ribosomiale inibendo la sintesi peptidica. Sintesi proteica Usi terapeutici I macrolidi sono usati per trattare le faringiti, tonsilliti, sinusiti, bronchiti, polmoniti, infezioni cutanee di malati di AIDS. Possono anche essere usai nei casi di legionella. Paragoni di MIC tra macrolidi Effetti Collaterali L’effetto collaterale più comune nei macrolidi è la diarrea, con nausea dolore addominale e vomito. Gli effetti meno comuni sono mal di testa, rash cutanei e alterazione del gusto. Gli effetti più gravi sono itterizia e insufficienza renale. Controindicazioni La claritromicina non deve essere usata in casi di soggetti con epatopatie e ridotta funzionalità renale. Inoltre in alcuni pazienti può portare al prolungamento del QT, bradicardia o riduzione dei livelli di potassio e magnesio. La claritromicina interagisce con molti farmaci e quindi deve essere considerata una possibile riduzione/aumento dell’atività di questi farmaci. Clarithromycin inhibits the middle to late stage of the influenza virus replication cycle, resulting in inhibition of progeny virus production from the infected cells. Macrolides could mediate this effect by inhibiting intracellular hemagglutinin HA0 proteolisis. The inhibitory effects on influenza virus replication of macrolides has been known since the 80s and have been observed for other viruses such as Sindibis virus. Resistance to macrolides in S. pneumoniae is mediated by 2 major mechanisms: 1) target modification caused by a ribosomal methylase encoded by the erm(B) gene or 2) drug efflux encoded by the mef(A) gene. High-level macrolide resistance (MIC required to inhibit growth in 90% of organisms [MIC90] >32 μg/mL) is usually associated with erm(B), whereas mef(A)-mediated resistance, the most prevalent mechanism in the United States, usually results in lower-level resistance (MIC90 1–4 μg/mL). Cochrane Database Syst Rev. 2004;(4):CD001954. Azithromycin for acute lower respiratory tract infections. Panpanich R, Lerttrakarnnon P, Laopaiboon M. Acute lower respiratory tract infections (LRTI) range from acute bronchitis and acute exacerbations of chronic bronchitis to pneumonia. There is unclear evidence that azithromycin is superior to amoxicillin or amoxyclav in treating acute LRTI. In patients with acute bronchitis of a suspected bacterial cause, azithromycin tends to be more effective in terms of lower incidence of treatment failure and adverse events than amoxicillin or amoxyclav. Lincosamidi Le lincosamidi (es. lincomicina, clindamicina) sono una classe di farmaci che legano la porzione 23s della subunità 50s dei batteri ribosomi inibendo il prolungamento del peptide attraverso l’inibizione della reazione di trans-peptidizzazione. Spettro d’azione antimicrobico La lincomicina possiede lo stesso spettro d’azione antimicrobico della claritromicina Clindamicina Lincomicina Macrolide, Lincosamidi e streptogramine (MLS) antibiotici. Continua............ Streptogramine Le Streptogramine sono degli antibiotici battericidi appartenenti a due categorie: 1) Streptogramina A : Dalfopristina 2) Streptogramina B: Quintupristina Streptogramina A dalfopristina Streptogramina B Meccanismo di azione Le streptogramine vengono somministrate insieme in un rapporto 30:70. Esse inibiscono la sintesi proteica in maniera sinergistica. La quinupristina si lega alla subunità 50s prevenendo l’allungamento della catena peptidica mentre la dalfopristina si lega ad un sito vicino alla subunità 50s ribosomiale aumentando il legame della quinupristina al ribosoma. Usi terapeutici Quinupristina/dalfopristina (Synercid) sono la combinazione dei due antibiotici. Viene usata per trattare le infezioni da staphylococci e da batteri resistenti alla vancomycina. Non sono efficaci nelle infezioni da Enterococcus faecalis Effetti collaterali Dolore articolari e muscolari, nausea, vomito e diarrea, Rash cuanei Tetracicline Le Tetracicline sono degli antibiotici a largo spettro d’azione prodotti dal genus streptomices degli actinobatteri. They are broad-spectrum antibiotics effective against aerobic and anaerobic Gram-positive and Gram-negative bacteria as well as Richettsia, Mycoplasma, Chlamydia spp. and Legionella spp. Tetracicline in terapia 1) 2) 3) 4) Minociclina Doxiciclina Demeclociclina oxitetraciclina Spettro d’azione antibatterica 1) Neisserie gonorreae 2) Clamidia 3) Bacillus antracis 4) Yersinia pestis 5) Richettsie (tifo) 6) Brucella 7) Treponema Meccanismo di azione Le tetracicline sono una famiglia di antibiotici che inibiscono il legame dell’RNA transfer alla subunità ribosomiale 30s del batterio. Sono batteriostatiche. They inhibit bacterial protein synthesis by binding to the 30S bacterial ribosome and preventing access of aminoacyl tRNA to the acceptor (A) site on the mRNA-ribosome complex. Tetracyclines can also cause alterations in the cytoplasmic membrane leading to leakage of nucleotides and other compounds from the bacterial cell. At higher concentrations, tetracycline may inhibit protein synthesis in mammalian cells. Doxycycline and minocycline are semi-synthetic derivatives of tetracycline. These two compounds are more lipid soluble which gives them some advantages over tetracycline hydrochloride, such as a lower dosage regimen, prolonged serum half-life, super tissue fluid penetration and decreased gastrointestinal side-effects Resistenza batterica alle tetracicline Cautele, Controindicazioni ed effetti collaterali. Le tetracicline possono macchiare I denti in fase di sviluppo nei bambini. Le tetracicline sono inattivate dagli ioni Ca++, Al3+, Fe2+ e Zn2+ presenti nel latte e nei cibi. Sono inattivate dai comuni farmaci antiacidi. Danno fotosensibilità, quindi deve essere evitata l’esposizione al sole. Possono dare lupus eritematosus, epatiti e tinniti. Tetracicline e gravidanza-allattamento Le tetracicline non possono essere somministrate in donne gravide sia per problemi vero il feto che verso la madre (epatopatia). Le tetracicline passano nel latte materno percui sono da evitare anche nell’allattamento. PubMed-MEDLINE and EMBASE databases (1974–March 2009), reviewed reference citations from identified publications, researched antibiotic prescribing information, and corresponded with drug manufacturers. Case reports, case series, and both in vivo and in vitro clinical trials were evaluated for the following antibiotics: clindamycin, daptomycin, linezolid, quinupristindalfopristin,rifampin, tetracycline, doxycycline, minocycline, tigecycline, trimethoprim-sulfamethoxazole, and vancomycin. Information for the newer antibiotics (linezolid, quinupristin-dalfopristin, tigecycline, and daptomycin) was limited. Despite heterogeneity in the data for the older antibiotics (clindamycin, rifampin, tetracyclines, trimethoprim-sulfamethoxazole, and vancomycin), all appear to be relatively safe in the minimal quantities nursing infants ingest through breast milk. Although the risk to infants seems to be relatively low for most of the agents we explored, the paucity of data indicates a need for close monitoring of breastfed infants whose mothers are receiving an antibiotic for an MRSA skin and soft tissue infection. Nuovi recenti farmaci approvati dall'FDA. Expert Opin Investig Drugs. 2010 February ; 19(2): 215–234. Oxazolidinoni Linezolid Linezolid is a synthetic antibiotic, the first of the oxazolidinone class, used for the treatment of infections caused by multiresistant bacteria including streptococcus and methicillin-resistant Staphylococcus aureus (MRSA). Meccanismo di azione The drug works by inhibiting the initiation of bacterial protein synthesis Glicilcicline Glycylcyclines are the newest member in the tetracycline class of antibacterial drugs and one member (tigecycline) was approved by the FDA (June 2005) 5 for complicated skin and skin structure infections and also for intraabdominal infection. The novelty about glycylcyclines is their ability to subvert the common tetracycline resistance mechanisms acquired by genetically mobile element encoding the tet genes. The two major resistance mechanisms are tetracycline efflux pumps or ribosomal protection. The compound with t-butylglycylamido moiety at position 9 of minocycline (tigecycline) exhibits potent antibacterial activity and also potency against tetracycline resistant bacteria. Mechanism of Action The glycylcyclines bind with a 5-fold to 100-fold higher affinity to 30S and and 70S ribosomes, respectively, than tetracyclines and also inhibit protein synthesis of Tet(M) and Tet(O) tetracycline resistant bacteria. Protein synthesis inhibition by tigecycline is 20-fold more efficient than tetracycline. tigecycline Ann Pharmacother. 2013 Mar;47(3):368-79. Cethromycin: a new ketolide antibiotic. Mansour H, Chahine EB, Karaoui LR, El-Lababidi RM. Source College of Pharmacy, University of Florida, FL, USA. [email protected] Abstract OBJECTIVE: To review the pharmacology, chemistry, microbiology, in vitro susceptibility, mechanism of resistance, pharmacokinetics, pharmacodynamics, clinical efficacy, safety, drug interactions, dosage, and administration of cethromycin, a new ketolide antibiotic. DATA SOURCES: Literature was obtained through searching PubMed (1950-October 2012), International Pharmaceutical Abstracts (1970-October 2012), and a bibliographic review of published articles. Search terms included cethromycin, ABT-773, ketolide antibiotic, and community-acquired pneumonia. STUDY SELECTION AND DATA EXTRACTION: All available in vitro and preclinical studies, as well as Phase 1, 2, and 3 clinical studies published in English were evaluated to summarize the pharmacology, chemistry, microbiology, efficacy, and safety of cethromycin in the treatment of respiratory tract infections. DATA SYNTHESIS: Cethromycin, a new ketolide, has a similar mechanism of action to telithromycin with an apparently better safety profile. Cethromycin displays in vitro activity against selected gram-positive, gram-negative, and atypical bacteria. The proposed indication of cethromycin is treatment of mild to moderate community-acquired bacterial pneumonia in patients aged 18 years or older. Based on clinical studies, the recommended dose is 300 mg orally once a day without regard to meals. Cethromycin has an orphan drug designation for tularemia, plague, and anthrax prophylaxis. The Food and Drug Administration denied approval for the treatment of community-acquired pneumonia in 2009; a recent noninferiority trial showed comparable efficacy between cethromycin and clarithromycin. Preliminary data on adverse effects suggest that cethromycin is safe and gastrointestinal adverse effects appear to be dose-related. CONCLUSIONS: Cethromycin appears to be a promising ketolide for the treatment of mild to moderate community-acquired pneumonia. It was denied approval by the FDA in 2009 pending more evidence to show its efficacy, with more recent studies showing its noninferiority to antibiotics for the same indication. PMID: 23463743
Scarica